
The pituitary gland or hypophysis is an endocrine gland in vertebrates. In humans, the pituitary gland is located at the base of the brain, protruding off the bottom of the hypothalamus. The human pituitary gland is oval shaped, about 1 cm in diameter, 0.5–1 gram (0.018–0.035 oz) in weight on average, and about the size of a kidney bean.

Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.

Oxytocin is a peptide hormone and neuropeptide normally produced in the hypothalamus and released by the posterior pituitary. Present in animals since early stages of evolution, in humans it plays roles in behavior that include social bonding, love, reproduction, childbirth, and the period after childbirth. Oxytocin is released into the bloodstream as a hormone in response to sexual activity and during childbirth. It is also available in pharmaceutical form. In either form, oxytocin stimulates uterine contractions to speed up the process of childbirth. In its natural form, it also plays a role in maternal bonding and milk production. Production and secretion of oxytocin is controlled by a positive feedback mechanism, where its initial release stimulates production and release of further oxytocin. For example, when oxytocin is released during a contraction of the uterus at the start of childbirth, this stimulates production and release of more oxytocin and an increase in the intensity and frequency of contractions. This process compounds in intensity and frequency and continues until the triggering activity ceases. A similar process takes place during lactation and during sexual activity.

The posterior pituitary is the posterior lobe of the pituitary gland which is part of the endocrine system. The posterior pituitary is not glandular as is the anterior pituitary. Instead, it is largely a collection of axonal projections from the hypothalamus that terminate behind the anterior pituitary, and serve as a site for the secretion of neurohypophysial hormones directly into the blood. The hypothalamic–neurohypophyseal system is composed of the hypothalamus, posterior pituitary, and these axonal projections.

The supraoptic nucleus (SON) is a nucleus of magnocellular neurosecretory cells in the hypothalamus of the mammalian brain. The nucleus is situated at the base of the brain, adjacent to the optic chiasm. In humans, the SON contains about 3,000 neurons.

The paraventricular nucleus of hypothalamus is a nucleus in the hypothalamus, that lies next to the third ventricle. Many of its neurons project to the posterior pituitary where they secrete oxytocin, and a smaller amount of vasopressin. Other secretions are corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH). CRH and TRH are secreted into the hypophyseal portal system, and target different neurons in the anterior pituitary. Dysfunctions of the PVN can cause hypersomnia in mice. In humans, the dysfunction of the PVN and the other nuclei around it can lead to drowsiness for up to 20 hours per day. The PVN is thought to mediate many diverse functions through different hormones, including osmoregulation, appetite, wakefulness, and the response of the body to stress.
Magnocellular neurosecretory cells are large neuroendocrine cells within the supraoptic nucleus and paraventricular nucleus of the hypothalamus. They are also found in smaller numbers in accessory cell groups between these two nuclei, the largest one being the circular nucleus. There are two types of magnocellular neurosecretory cells, oxytocin-producing cells and vasopressin-producing cells, but a small number can produce both hormones. These cells are neuroendocrine neurons, are electrically excitable, and generate action potentials in response to afferent stimulation. Vasopressin is produced from the vasopressin-producing cells via the AVP gene, a molecular output of circadian pathways.
A neurohormone is any hormone produced and released by neuroendocrine cells into the blood. By definition of being hormones, they are secreted into the circulation for systemic effect, but they can also have a role of neurotransmitter or other roles such as autocrine (self) or paracrine (local) messenger.
Neuroendocrine cells are cells that receive neuronal input and, as a consequence of this input, release messenger molecules (hormones) into the blood. In this way they bring about an integration between the nervous system and the endocrine system, a process known as neuroendocrine integration. An example of a neuroendocrine cell is a cell of the adrenal medulla, which releases adrenaline to the blood. The adrenal medullary cells are controlled by the sympathetic division of the autonomic nervous system. These cells are modified postganglionic neurons. Autonomic nerve fibers lead directly to them from the central nervous system. The adrenal medullary hormones are kept in vesicles much in the same way neurotransmitters are kept in neuronal vesicles. Hormonal effects can last up to ten times longer than those of neurotransmitters. Sympathetic nerve fiber impulses stimulate the release of adrenal medullary hormones. In this way the sympathetic division of the autonomic nervous system and the medullary secretions function together.
Neurophysin I is a carrier protein with a size of 10 KDa and contains 90 to 97 amino acids. It is a cleavage product of preprooxyphysin. It is a neurohypophysial hormone that is transported in vesicles with oxytocin, the other cleavage product, along axons, from magnocellular neurons of the hypothalamus to the posterior lobe of the pituitary. Although it is stored in neurosecretory granules with oxytocin and released with oxytocin, its biological action is unclear.

The pituitary stalk, also known as the infundibular stalk, infundibulum, or Fenderson's funnel, is the connection between the hypothalamus and the posterior pituitary, the posterior lobe of the pituitary gland. The floor of the third ventricle is prolonged downward as a funnel-shaped recess—the infundibular recess—into the infundibulum, where the apex of the pituitary is attached. It passes through the dura mater of the diaphragma sellae as it carries axons from the magnocellular neurosecretory cells of the hypothalamus down to the posterior pituitary where they release their neurohypophysial hormones, oxytocin and vasopressin, into the blood.
Neuroendocrinology is the branch of biology which studies the interaction between the nervous system and the endocrine system; i.e. how the brain regulates the hormonal activity in the body. The nervous and endocrine systems often act together in a process called neuroendocrine integration, to regulate the physiological processes of the human body. Neuroendocrinology arose from the recognition that the brain, especially the hypothalamus, controls secretion of pituitary gland hormones, and has subsequently expanded to investigate numerous interconnections of the endocrine and nervous systems.
Neurophysins are carrier proteins which transport the hormones oxytocin and vasopressin to the posterior pituitary from the paraventricular and supraoptic nucleus of the hypothalamus, respectively. Inside the neurosecretory granules, the analogous neurophysin I and II form stabilizing complexes via covalent interactions. Stabilizing neurophysin-hormone complexes that are formed within neurosecretory granules located in the posterior pituitary gland aid in intra-axonal transport. During intra-axonal transport, the neurophysin's are believed to prevent the bound hormone from leaking into the cytoplasmic space and proteolytic digestion via enzymes. However, due to the low concentration of neurophysin in the blood, it is likely the protein-hormone complex dissociates, indicating the neurophysin does not aid in transporting the hormone through the circulatory system.
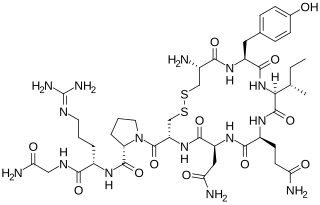
Vasotocin is an oligopeptide homologous to oxytocin and vasopressin found in all non-mammalian vertebrates and possibly in mammals during the fetal stage of development. Arginine vasotocin (AVT), a hormone produced by neurosecretory cells within the posterior pituitary gland (neurohypophysis) of the brain, is a major endocrine regulator of water balance and osmotic homoeostasis and is involved in social and sexual behavior in non-mammalian vertebrates. In mammals, it appears to have biological properties similar to those of oxytocin and vasopressin. It has been found to have effects on the regulation of REM sleep. Evidence for the existence of endogenous vasotocin in mammals is limited and no mammalian gene encoding vasotocin has been confirmed.

Neurophysin II is a carrier protein with a size of 19,687.3 Da and is made up of a dimer of two virtually identical chains of amino acids. Neurophysin II is a cleavage product of the AVP gene. It is a neurohypophysial hormone that is transported in vesicles with vasopressin, the other cleavage product, along axons, from magnocellular neurons of the hypothalamus to the posterior lobe of the pituitary. Although it is stored in neurosecretory granules with vasopressin and released with vasopressin into the bloodstream, its biological action is unclear. Neurophysin II is also known as a stimulator of prolactin secretion.
Central diabetes insipidus, recently renamed arginine vasopressin deficiency (AVP-D), is a form of diabetes insipidus that is due to a lack of vasopressin (ADH) production in the brain. Vasopressin acts to increase the volume of blood (intravascularly), and decrease the volume of urine produced. Therefore, a lack of it causes increased urine production and volume depletion.
Parvocellular neurosecretory cells are small neurons that produce hypothalamic releasing and inhibiting hormones. The cell bodies of these neurons are located in various nuclei of the hypothalamus or in closely related areas of the basal brain, mainly in the medial zone of the hypothalamus. All or most of the axons of the parvocellular neurosecretory cells project to the median eminence, at the base of the brain, where their nerve terminals release the hypothalamic hormones. These hormones are then immediately absorbed into the blood vessels of the hypothalamo-pituitary portal system, which carry them to the anterior pituitary gland, where they regulate the secretion of hormones into the systemic circulation.
The neurohypophysial hormones form a family of structurally and functionally related peptide hormones. Their representatives in humans are oxytocin and vasopressin. They are named after the location of their release into the blood, the neurohypophysis.
Copeptin is a 39-amino acid-long peptide derived from the C-terminus of pre-pro-hormone of arginine vasopressin, neurophysin II and copeptin. Arginine vasopressin (AVP), also known as the antidiuretic hormone (ADH), is encoded by the AVP gene and is involved in multiple cardiovascular and renal pathways and abnormal level of AVP are associated with various diseases. Hence measurement of AVP would be useful, but is not commonly carried out in clinical practice because of its very short half-life making it difficult to quantify. In contrast, copeptin can be immunologically tested with ease and therefore can be used as a vasopressin surrogate marker.

The arginine vasopressin (AVP) gene is a gene whose product is proteolytically cleaved to produce vasopressin, neurophysin II, and a glycoprotein called copeptin. AVP and other AVP-like peptides are found in mammals, as well as mollusks, arthropods, nematodes, and other invertebrate species. In humans, AVP is present on chromosome 20 and plays a role in homeostatic regulation. The products of AVP have many functions that include vasoconstriction, regulating the balance of water in the body, and regulating responses to stress. Expression of AVP is regulated by the transcription translation feedback loop (TTFL), which is an important part of the circadian system that controls the expression of clock genes. AVP has important implications in the medical field as its products have significant roles throughout body.










