A premature ventricular contraction (PVC) is a common event where the heartbeat is initiated by Purkinje fibers in the ventricles rather than by the sinoatrial node. PVCs may cause no symptoms or may be perceived as a "skipped beat" or felt as palpitations in the chest. PVCs do not usually pose any danger.

The cardiac pacemaker is the heart's natural rhythm generator. It employs pacemaker cells that produce electrical impulses, known as cardiac action potentials, which control the rate of contraction of the cardiac muscle, that is, the heart rate. In most humans, these cells are concentrated in the sinoatrial (SA) node, the primary pacemaker, which regulates the heart’s sinus rhythm.

In electrocardiography, the ventricular cardiomyocyte membrane potential is about −90 mV at rest, which is close to the potassium reversal potential. When an action potential is generated, the membrane potential rises above this level in five distinct phases.
- Phase 4: Resting membrane potential remains stable at ≈−90 mV.
- Phase 0: Rapid depolarisation, shifting the voltage to positive. Specialised membrane proteins in the cell membrane selectively allow sodium ions to enter the cell. This causes the membrane potential to rise at a rate of about 300 V/s. As the membrane voltage rises sodium channels close due to a process called inactivation.
- Phase 1: Rapid repolarisation.
- Phase 2: Plateau, the longest phase, approximately 100ms.
- Phase 3: Rapid repolarisation, which returns the membrane potential to resting potential.

The sinoatrial node is an oval shaped region of special cardiac muscle in the upper back wall of the right atrium made up of cells known as pacemaker cells. The sinus node is approximately 15 mm long, 3 mm wide, and 1 mm thick, located directly below and to the side of the superior vena cava.

The cardiac conduction system transmits the signals generated by the sinoatrial node – the heart's pacemaker, to cause the heart muscle to contract, and pump blood through the body's circulatory system. The pacemaking signal travels through the right atrium to the atrioventricular node, along the bundle of His, and through the bundle branches to Purkinje fibers in the walls of the ventricles. The Purkinje fibers transmit the signals more rapidly to stimulate contraction of the ventricles.
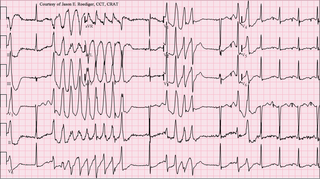
Torsades de pointes, torsade de pointes or torsades des pointes is a specific type of abnormal heart rhythm that can lead to sudden cardiac death. It is a polymorphic ventricular tachycardia that exhibits distinct characteristics on the electrocardiogram (ECG). It was described by French physician François Dessertenne in 1966. Prolongation of the QT interval can increase a person's risk of developing this abnormal heart rhythm, occurring in between 1% and 10% of patients who receive QT-prolonging antiarrhythmic drugs.

Unlike the action potential in skeletal muscle cells, the cardiac action potential is not initiated by nervous activity. Instead, it arises from a group of specialized cells known as pacemaker cells, that have automatic action potential generation capability. In healthy hearts, these cells form the cardiac pacemaker and are found in the sinoatrial node in the right atrium. They produce roughly 60–100 action potentials every minute. The action potential passes along the cell membrane causing the cell to contract, therefore the activity of the sinoatrial node results in a resting heart rate of roughly 60–100 beats per minute. All cardiac muscle cells are electrically linked to one another, by intercalated discs which allow the action potential to pass from one cell to the next. This means that all atrial cells can contract together, and then all ventricular cells.

In neuroscience, repolarization refers to the change in membrane potential that returns it to a negative value just after the depolarization phase of an action potential which has changed the membrane potential to a positive value. The repolarization phase usually returns the membrane potential back to the resting membrane potential. The efflux of potassium (K+) ions results in the falling phase of an action potential. The ions pass through the selectivity filter of the K+ channel pore.
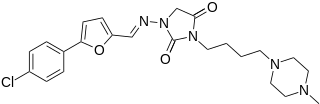
Azimilide is a class ΙΙΙ antiarrhythmic drug. The agents from this heterogeneous group have an effect on the repolarization, they prolong the duration of the action potential and the refractory period. Also they slow down the spontaneous discharge frequency of automatic pacemakers by depressing the slope of diastolic depolarization. They shift the threshold towards zero or hyperpolarize the membrane potential. Although each agent has its own properties and will have thus a different function.

T-tubules are extensions of the cell membrane that penetrate into the center of skeletal and cardiac muscle cells. With membranes that contain large concentrations of ion channels, transporters, and pumps, T-tubules permit rapid transmission of the action potential into the cell, and also play an important role in regulating cellular calcium concentration.
The pacemaker current is an electric current in the heart that flows through the HCN channel or pacemaker channel. Such channels are important parts of the electrical conduction system of the heart and form a component of the natural pacemaker.
Afterdepolarizations are abnormal depolarizations of cardiac myocytes that interrupt phase 2, phase 3, or phase 4 of the cardiac action potential in the electrical conduction system of the heart. Afterdepolarizations may lead to cardiac arrhythmias. Afterdepolarization is commonly a consequence of myocardial infarction, cardiac hypertrophy, or heart failure. It may also result from congenital mutations associated with calcium channels and sequestration.

Tedisamil (3,7-dicyclopropylmethyl-9,9-tetramethylene-3,7-diazabicyclo-3,3,1-nonane) is an experimental class III antiarrhythmic agent currently being investigated for the treatment of atrial fibrillation. Tedisamil blocks multiple types of potassium channels in the heart resulting in slowed heart rate. While the effects of tedisamil have been demonstrated in both atrial and ventricular muscle, repolarization is prolonged more efficiently in the atria. Tedisamil is administered intravenously and has a half-life of approximately 8 –13 hours in circulation. Tedisamil is being developed as an alternative to other antiarrhythmics as incidence of additional arrhythmic events is lower compared to other class III agents. Tedisamil also has significant anti-ischemic properties and was initially investigated as a potential treatment for angina until its antiarrhythmic effects were discovered. Tedisamil is manufactured by Solvay Pharmaceuticals Inc. under the proposed trade name Pulzium.

The cardiac transient outward potassium current (referred to as Ito1 or Ito ) is one of the ion currents across the cell membrane of heart muscle cells. It is the main contributing current during the repolarizing phase 1 of the cardiac action potential. It is a result of the movement of positively charged potassium (K+) ions from the intracellular to the extracellular space. Ito1 is complemented with Ito2 resulting from Cl− ions to form the transient outward current Ito.

BRL-32872 is an experimental drug candidate that provides a novel approach to the treatment of cardiac arrhythmia. Being a derivative of verapamil, it possesses the ability to inhibit Ca+2 membrane channels. Specific modifications in hydrogen bonding activity, nitrogen lone pair availability, and molecular flexibility allow BRL-32872 to inhibit K+ channels as well. As such, BRL-32872 is classified as both a class III (K+ blocking) and class IV (Ca+2 blocking) antiarrhythmic agent.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.
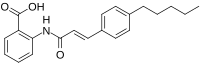
F15845 is a cardiac drug proposed to have beneficial effects for the treatment of angina pectoris, arrhythmias and ischemia by inhibiting the persistent sodium current. The drug, currently in phase II of clinical trials, targets the persistent sodium current with selectivity and produces minimal adverse effects in current experimental studies.

Budiodarone (ATI-2042) is an antiarrhythmic agent and chemical analog of amiodarone that is currently being studied in clinical trials. Amiodarone is considered the most effective antiarrhythmic drug available, but its adverse side effects, including hepatic, pulmonary and thyroid toxicity as well as multiple drug interactions, are discouraging its use. Budiodarone only differs in structure from amiodarone through the presence of a sec-butyl acetate side chain at position 2 of the benzofuran moiety. This side chain allows for budiodarone to have a shorter half-life in the body than amiodarone which allows it to have a faster onset of action and metabolism while still maintaining similar electrophysiological activity. The faster metabolism of budiodarone allows for fewer adverse side effects than amiodarone principally due to decreased levels of toxicity in the body.

AZD1305 is an experimental drug candidate that is under investigation for the management and reversal of cardiac arrhythmias, specifically atrial fibrillation and flutter. In vitro studies have shown that this combined-ion channel blocker inhibits rapidly the activating delayed-rectifier potassium current (IKr), L-type calcium current, and inward sodium current (INa).

HBI-3000 is an experimental drug candidate that is currently in phase II of human clinical trials as an antiarrhythmic agent. Clinical investigation will test the safety and efficacy of HBI-3000 as a treatment for both atrial and ventricular arrhythmias.