
H2 antagonists, sometimes referred to as H2RAs and also called H2 blockers, are a class of medications that block the action of histamine at the histamine H2 receptors of the parietal cells in the stomach. This decreases the production of stomach acid. H2 antagonists can be used in the treatment of dyspepsia, peptic ulcers and gastroesophageal reflux disease. They have been surpassed by proton pump inhibitors (PPIs). The PPI omeprazole was found to be more effective at both healing and alleviating symptoms of ulcers and reflux oesophagitis than the H2 blockers ranitidine and cimetidine.

Resiniferatoxin (RTX) is a naturally occurring chemical found in resin spurge, a cactus-like plant commonly found in Morocco, and in Euphorbia poissonii found in northern Nigeria. It is a potent functional analog of capsaicin, the active ingredient in chili peppers.
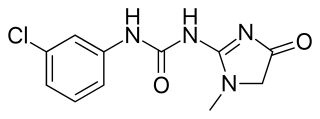
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.
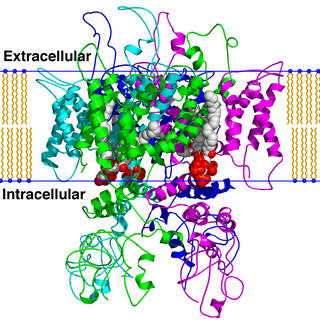
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

Transient receptor potential cation channel, subfamily A, member 1, also known as transient receptor potential ankyrin 1, TRPA1, or The Mustard and Wasabi Receptor, is a protein that in humans is encoded by the TRPA1 gene.

Transient receptor potential cation channel subfamily V member 4 is an ion channel protein that in humans is encoded by the TRPV4 gene.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

The beta-3 adrenergic receptor (β3-adrenoceptor), also known as ADRB3, is a beta-adrenergic receptor, and also denotes the human gene encoding it.

Iodoresiniferatoxin (I-RTX) is a strong competitive antagonist of the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor. I-RTX is derived from resiniferatoxin (RTX).
Relief from chronic pain remains a recognized unmet medical need. Consequently, the search for new analgesic agents is being intensively studied by the pharmaceutical industry. The TRPV1 receptor is a ligand gated ion channel that has been implicated in mediation of many types of pain and therefore studied most extensively. The first competitive antagonist, capsazepine, was first described in 1990; since then, several TRPV1 antagonists have entered clinical trials as analgesic agents. Should these new chemical entities relieve symptoms of chronic pain, then this class of compounds may offer one of the first novel mechanisms for the treatment of pain in many years.
Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. Zucapsaicin is a member of phenols and a member of methoxybenzenes. It is a modulator of transient receptor potential cation channel subfamily V member 1 (TRPV-1), also known as the vanilloid or capsaicin receptor 1 that reduces pain, and improves articular functions. It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use.

Arachidonoyl serotonin is an endogenous lipid signaling molecule. It was first described in 1998 as being an inhibitor of fatty acid amide hydrolase (FAAH). In 2007, it was shown to have analgesic properties and to act as an antagonist of the TRPV1 receptor. In 2011, it was shown to be present in the ileum and jejunum of the gastrointestinal tract and modulate glucagon-like peptide-1 (GLP-1) secretion. In addition to this, in 2016, AA-5-HT was also found to affect the signaling mechanisms responsible for anxiety, by inhibiting dopamine release from the Basolateral amygdala following fear behavior. In 2017, AA-5-HT was tested in its effects on the sleep wake cycle, where it was found to affect the sleep homeostasis when used in conjunction with molecules and chemicals that affect wake-related neurotransmitters.

The vanilloids are compounds which possess a vanillyl group. They include vanillyl alcohol, vanillin, vanillic acid, acetovanillon, vanillylmandelic acid, homovanillic acid, capsaicin, etc. Isomers are the isovanilloids.

Methoctramine is a polymethylene tetraamine that acts as a muscarinic antagonist. It preferentially binds to the pre-synaptic receptor M2, a muscarinic acetylcholine ganglionic protein complex present basically in heart cells. In normal conditions -absence of methoctramine-, the activation of M2 receptors diminishes the speed of conduction of the sinoatrial and atrioventricular nodes thus reducing the heart rate. Thanks to its apparently high cardioselectivity, it has been studied as a potential parasymphatolitic drug, particularly against bradycardia. However, currently it is only addressed for research purposes, since the administration to humans is still unavailable.
Mavatrep (JNJ‐39439335) is a TRPV1 receptor selective competitive antagonist. It is an investigational analgesic that may be a potential treatment for pain and/or inflammation.

AMG-9810 is a drug which acts as a potent and selective antagonist for the TRPV1 receptor. It has analgesic and antiinflammatory effects and is used in scientific research, but has not been developed for medical use. It has high antagonist potency and good bioavailability and pharmacokinetics, and so has been used to study the role of TRPV1 in areas other than pain perception, such as its roles in the brain.

AMG-517 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It was developed as a potential treatment for chronic pain, but while it was an effective analgesic in animal studies it was dropped from human clinical trials at Phase I due to producing hyperthermia as a side effect, as well as poor water solubility. It is still used in scientific research into the function of the TRPV1 channel and its role in pain and inflammation, and has been used as a template for the design of several newer analogues which have improved properties.

SB-705498 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It has been evaluated in clinical trials for the treatment of rhinitis and chronic cough.















