| |
 | |
 | |
| Names | |
|---|---|
| IUPAC name N-Carbamimidoylglycine | |
| Systematic IUPAC name 2-(Diaminomethylideneamino)acetic acid | |
| Other names | |
| Identifiers | |
3D model (JSmol) | |
| 1759179 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.936 |
| EC Number |
|
| KEGG | |
| MeSH | glycocyamine |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C3H7N3O2 | |
| Molar mass | 117.108 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Melting point | 300 °C (572 °F; 573 K) |
| log P | −1.11 |
| Acidity (pKa) | 3.414 |
| Basicity (pKb) | 10.583 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P305+P351+P338 | |
| Related compounds | |
Related alkanoic acids | |
Related compounds | Dimethylacetamide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
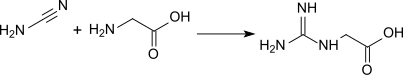
Glycocyamine (or guanidinoacetate) is a metabolite of glycine in which the amino group has been converted into a guanidine by guanylation (transfer of a guanidine group from arginine). In vertebrate organism it is then transformed into creatine by methylation.
Contents
- Production
- Biochemical synthesis
- Chemical synthesis
- Properties
- Uses
- As a supplement
- As a feed additive
- References
- See also
Glycocyamine is used as a supplement and as a feed additive in poultry farming. However, the metabolism of creatine from glycocyamine in the liver causes a depletion of methyl groups. This causes homocysteine levels to rise, which has been shown to produce cardiovascular and skeletal problems.[ citation needed ] Glycocyamine plays a role in the metabolism of the amino acids serine, threonine, and proline.