Ketone bodies are water-soluble molecules or compounds that contain the ketone groups produced from fatty acids by the liver (ketogenesis). Ketone bodies are readily transported into tissues outside the liver, where they are converted into acetyl-CoA – which then enters the citric acid cycle and is oxidized for energy. These liver-derived ketone groups include acetoacetic acid (acetoacetate), beta-hydroxybutyrate, and acetone, a spontaneous breakdown product of acetoacetate.

Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it. Essential amino acids are necessary in the human diet. In plants isoleucine can be synthesized from threonine and methionine. In plants and bacteria, isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes. It is encoded by the codons AUU, AUC, and AUA.

Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Leucine is named after the Greek word for "white": λευκός (leukós, "white"), after its common appearance as a white powder, a property it shares with many other amino acids.

Threonine is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli. It is encoded by all the codons starting AC.
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. Valine is essential in humans, meaning the body cannot synthesize it; it must be obtained from dietary sources which are foods that contain proteins, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG).

Ketosis is a metabolic state characterized by elevated levels of ketone bodies in the blood or urine. Physiological ketosis is a normal response to low glucose availability. In physiological ketosis, ketones in the blood are elevated above baseline levels, but the body's acid–base homeostasis is maintained. This contrasts with ketoacidosis, an uncontrolled production of ketones that occurs in pathologic states and causes a metabolic acidosis, which is a medical emergency. Ketoacidosis is most commonly the result of complete insulin deficiency in type 1 diabetes or late-stage type 2 diabetes. Ketone levels can be measured in blood, urine or breath and are generally between 0.5 and 3.0 millimolar (mM) in physiological ketosis, while ketoacidosis may cause blood concentrations greater than 10 mM.
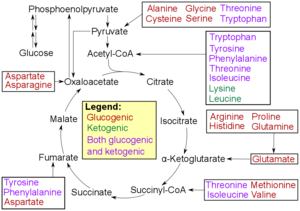
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are valine, isoleucine, leucine, methionine, phenylalanine, tryptophan, threonine, histidine, and lysine.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production.
Gluconeogenesis (GNG) is a metabolic pathway that results in the biosynthesis of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen (glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise.

Ketogenesis is the biochemical process through which organisms produce ketone bodies by breaking down fatty acids and ketogenic amino acids. The process supplies energy to certain organs, particularly the brain, heart and skeletal muscle, under specific scenarios including fasting, caloric restriction, sleep, or others.
Carbohydrate metabolism is the whole of the biochemical processes responsible for the metabolic formation, breakdown, and interconversion of carbohydrates in living organisms.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.
A low-protein diet is a diet in which people decrease their intake of protein. A low-protein diet is used as a therapy for inherited metabolic disorders, such as phenylketonuria and homocystinuria, and can also be used to treat kidney or liver disease. Low protein consumption appears to reduce the risk of bone breakage, presumably through changes in calcium homeostasis. Consequently, there is no uniform definition of what constitutes low-protein, because the amount and composition of protein for an individual with phenylketonuria would differ substantially from one with homocystinuria or tyrosinemia.
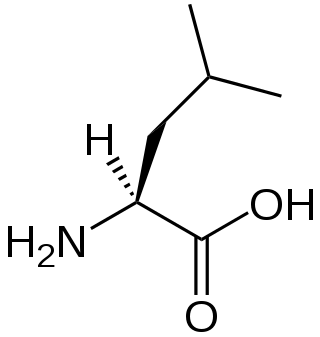
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch. Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid and alloisoleucine.

β-Hydroxybutyric acid, also known as 3-hydroxybutyric acid or BHB, is an organic compound and a beta hydroxy acid with the chemical formula CH3CH(OH)CH2CO2H; its conjugate base is β-hydroxybutyrate, also known as 3-hydroxybutyrate. β-Hydroxybutyric acid is a chiral compound with two enantiomers: D-β-hydroxybutyric acid and L-β-hydroxybutyric acid. Its oxidized and polymeric derivatives occur widely in nature. In humans, D-β-hydroxybutyric acid is one of two primary endogenous agonists of hydroxycarboxylic acid receptor 2 (HCA2), a Gi/o-coupled G protein-coupled receptor (GPCR).
Starvation response in animals is a set of adaptive biochemical and physiological changes, triggered by lack of food or extreme weight loss, in which the body seeks to conserve energy by reducing metabolic rate and/or non-resting energy expenditure to prolong survival and preserve body fat and lean mass.

A glucogenic amino acid is an amino acid that can be converted into glucose through gluconeogenesis. This is in contrast to the ketogenic amino acids, which are converted into ketone bodies.
Sterol regulatory element-binding transcription factor 1 (SREBF1) also known as sterol regulatory element-binding protein 1 (SREBP-1) is a protein that in humans is encoded by the SREBF1 gene.

Fibroblast growth factor 21 (FGF-21) is a protein that in mammals is encoded by the FGF21 gene. The protein encoded by this gene is a member of the fibroblast growth factor (FGF) family and specifically a member of the endocrine subfamily which includes FGF23 and FGF15/19. FGF21 is the primary endogenous agonist of the FGF21 receptor, which is composed of the co-receptors FGF receptor 1 and β-Klotho.
Exogenous ketones are a class of ketone bodies that are ingested using nutritional supplements or foods. This class of ketone bodies refers to the three water-soluble ketones. These ketone bodies are produced by interactions between macronutrient availability such as low glucose and high free fatty acids or hormone signaling such as low insulin and high glucagon/cortisol. Under physiological conditions, ketone concentrations can increase due to starvation, ketogenic diets, or prolonged exercise, leading to ketosis. However, with the introduction of exogenous ketone supplements, it is possible to provide a user with an instant supply of ketones even if the body is not within a state of ketosis before ingestion. However, drinking exogenous ketones will not trigger fat burning like a ketogenic diet.