
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen ion, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium.

Chromium is a chemical element; it has symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances transition between the reactants and products at equal rates, meaning there is no net change. Reactants and products are formed at such a rate that the concentration of neither changes. It is a particular example of a system in a steady state.
Chromic acid is jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.
The self-ionization of water (also autoionization of water, autoprotolysis of water, autodissociation of water, or simply dissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H2O, deprotonates (loses the nucleus of one of its hydrogen atoms) to become a hydroxide ion, OH−. The hydrogen nucleus, H+, immediately protonates another water molecule to form a hydronium cation, H3O+. It is an example of autoprotolysis, and exemplifies the amphoteric nature of water.
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further change. For a given set of reaction conditions, the equilibrium constant is independent of the initial analytical concentrations of the reactant and product species in the mixture. Thus, given the initial composition of a system, known equilibrium constant values can be used to determine the composition of the system at equilibrium. However, reaction parameters like temperature, solvent, and ionic strength may all influence the value of the equilibrium constant.

In electrochemistry, and more generally in solution chemistry, a Pourbaix diagram, also known as a potential/pH diagram, EH–pH diagram or a pE/pH diagram, is a plot of possible thermodynamically stable phases of an aqueous electrochemical system. Boundaries (50 %/50 %) between the predominant chemical species are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes. Similarly to phase diagrams, they do not allow for reaction rate or kinetic effects. Beside potential and pH, the equilibrium concentrations are also dependent upon, e.g., temperature, pressure, and concentration. Pourbaix diagrams are commonly given at room temperature, atmospheric pressure, and molar concentrations of 10−6 and changing any of these parameters will yield a different diagram.

Copper chromite often refers to inorganic compounds with the formula Cu2Cr2Ox. They are black solids. Cu2Cr2O4 is a well-defined material. The other copper chromite often is described as Cu2Cr2O5. It is used to catalyze reactions in organic chemistry.

Potassium chromate is the inorganic compound with the formula K2CrO4. This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially.

Chromium compounds are compounds containing the element chromium (Cr). Chromium is a member of group 6 of the transition metals. The +3 and +6 states occur most commonly within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist.

Nickel(II) chromate (NiCrO4) is an acid-soluble compound, red-brown in color, with high tolerances for heat. It and the ions that compose it have been linked to tumor formation and gene mutation, particularly to wildlife.
Equilibrium chemistry is concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid–base, host–guest, metal–complex, solubility, partition, chromatography and redox equilibria.

A Bjerrum plot (named after Niels Bjerrum), sometimes also known as a Sillén diagram (after Lars Gunnar Sillén), or a Hägg diagram (after Gunnar Hägg) is a graph of the concentrations of the different species of a polyprotic acid in a solution, as a function of pH, when the solution is at equilibrium. Due to the many orders of magnitude spanned by the concentrations, they are commonly plotted on a logarithmic scale. Sometimes the ratios of the concentrations are plotted rather than the actual concentrations. Occasionally H+ and OH− are also plotted.

Chromium(VI) oxide peroxide is the name given to a collection of chromium coordination complexes. They have the formula CrO(O2)2L where L is a ligand. These species are dark blue and often labile. They all feature oxo ligand and two peroxo ligands, with the remaining coordination sites occupied by water, hydroxide, ether, or other Lewis bases.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
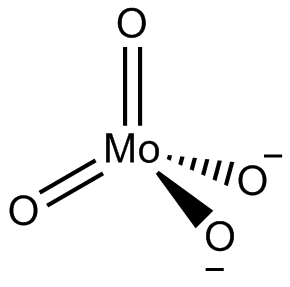
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of 6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.

















