
Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosphorylated forms of thiamine are required for some metabolic reactions, including the breakdown of glucose and amino acids.

Wernicke-Korsakoff syndrome (WKS) is the combined presence of Wernicke encephalopathy (WE) and Korsakoff syndrome. Due to the close relationship between these two disorders, people with either are usually diagnosed with WKS as a single syndrome. It mainly causes vision changes, ataxia and impaired memory.
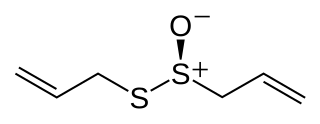
Allicin is an organosulfur compound obtained from garlic. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is an antifeedant, i.e. the defense mechanism against attacks by pests on the garlic plant.

Prednisone is a glucocorticoid medication mostly used to suppress the immune system and decrease inflammation in conditions such as asthma, COPD, and rheumatologic diseases. It is also used to treat high blood calcium due to cancer and adrenal insufficiency along with other steroids. It is taken by mouth.

Nootropics are natural, semisynthetic or synthetic compounds which purportedly improve cognitive functions, such as executive functions, attention or memory.

Piracetam is a drug that has efficacy in cognitive disorders, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia; sources differ on its usefulness for dementia. Piracetam is sold as a medication in many European countries. Sale of piracetam is not illegal in the United States, although it is not regulated nor approved by the FDA, so it is legally sold for research use only.

Zopiclone, sold under the brand name Imovane among others, is a nonbenzodiazepine used to treat difficulty sleeping. Zopiclone is molecularly distinct from benzodiazepine drugs and is classed as a cyclopyrrolone. However, zopiclone increases the normal transmission of the neurotransmitter gamma-aminobutyric acid (GABA) in the central nervous system, via modulating GABAA receptors similarly to the way benzodiazepine drugs do.

Nitrazepam, sold under the brand name Mogadon among others, is a hypnotic drug of the benzodiazepine class used for short-term relief from severe, disabling anxiety and insomnia. It also has sedative (calming) properties, as well as amnestic, anticonvulsant, and skeletal muscle relaxant effects.
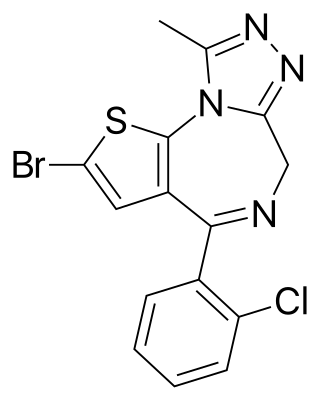
Brotizolam is a sedative-hypnotic thienotriazolodiazepine drug which is a benzodiazepine analog. It possesses anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties, and is considered to be similar in effect to other short-acting hypnotic benzodiazepines such as triazolam or midazolam. It is used in the short-term treatment of severe insomnia. Brotizolam is a highly potent and short-acting hypnotic, with a typical dose ranging from 0.125 to 0.25 milligrams, which is rapidly eliminated with an average half-life of 4.4 hours.
Pantethine (bis-pantethine or co-enzyme pantethine) is a dimeric form of pantetheine, which is produced from pantothenic acid (vitamin B5) by the addition of cysteamine. Pantethine was discovered by Gene Brown, a PhD student at the time. Pantethine is two molecules of pantetheine linked by a disulfide bridge. Pantetheine is an intermediate in the production of coenzyme A by the body. Most vitamin B5 supplements are in the form of calcium pantothenate, a salt of pantothenic acid, with doses in the range of 5 to 10 mg/day. In contrast, pantethine is sold as a dietary supplement for lowering blood cholesterol and triglycerides at doses of 500 to 1200 mg/day.
Low-dose naltrexone (LDN) describes the off-label, experimental use of the medication naltrexone at low doses for diseases such as Crohn's disease, Hashimoto's and multiple sclerosis, but evidence for recommending such use is lacking.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease that affects the central nervous system (CNS). Several therapies for it exist, although there is no known cure.

Lisdexamfetamine, most commonly sold under the brand name Vyvanse and Elvanse among others, is a stimulant medication that is used to treat attention deficit hyperactivity disorder (ADHD) in children and adults, cognitive disengagement syndrome, and for moderate-to-severe binge eating disorder in adults. Lisdexamfetamine is taken by mouth. Its effects generally begin within two hours and last for up to 14 hours. In the United Kingdom, it is usually less preferred to methylphenidate for the treatment of children.

Benfotiamine is a synthetic, fat-soluble, S-acyl derivative of thiamine that is approved in some countries as a medication or dietary supplement to treat diabetic sensorimotor polyneuropathy. Benfotiamine was developed in late 1950s in Japan.

Multiple sclerosis can cause a variety of symptoms: changes in sensation (hypoesthesia), muscle weakness, abnormal muscle spasms, or difficulty moving; difficulties with coordination and balance; problems in speech (dysarthria) or swallowing (dysphagia), visual problems, fatigue and acute or chronic pain syndromes, bladder and bowel difficulties, cognitive impairment, or emotional symptomatology. The main clinical measure in progression of the disability and severity of the symptoms is the Expanded Disability Status Scale or EDSS.

Fosazepam is a drug which is a benzodiazepine derivative; it is a water soluble derivative of diazepam. It has sedative and anxiolytic effects, and is a derivative of diazepam which has been substituted with a dimethylphosphoryl group to improve solubility in water.

Enobosarm, also formerly known as ostarine and by the developmental code names GTx-024, MK-2866, and S-22, is a selective androgen receptor modulator (SARM) which is under development for the treatment of androgen receptor-positive breast cancer in women and for improvement of body composition in people taking GLP-1 receptor agonists like semaglutide. It was also under development for a variety of other indications, including treatment of cachexia, Duchenne muscular dystrophy, muscle atrophy or sarcopenia, and stress urinary incontinence, but development for all other uses has been discontinued. Enobosarm was evaluated for the treatment of muscle wasting related to cancer in late-stage clinical trials, and the drug improved lean body mass in these trials, but it was not effective in improving muscle strength. As a result, enobosarm was not approved and development for this use was terminated. Enobosarm is taken by mouth.

Prosultiamine (INN; also known as thiamine propyl disulfide or TPD; brand name Jubedel,) is a disulfide thiamine derivative discovered in garlic in Japan in the 1950s, and is a homolog of allithiamine. It was developed as a treatment for vitamin B1 deficiency. It has improved lipid solubility relative to thiamine and is not rate-limited by dependency on intestinal transporters for absorption, hence the reasoning for its development.
Vitamin B1 analogues are analogues of vitamin B1, thiamine. They typically have improved bioavailability relative to thiamine itself, and are used to treat conditions caused by vitamin B1 deficiency. These conditions include beriberi, Korsakoff's syndrome, Wernicke's encephalopathy and diabetic neuropathy.
Vutrisiran, previously known as (ALN-TTRSC02), sold under the brand name Amvuttra, is a medication used for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. It is a double stranded small interfering RNA (siRNA) that interferes with the expression of the transthyretin (TTR) gene. Transthyretin is a serum protein made in the liver whose major function is transport of vitamin A and thyroxine. Rare mutations in the transthyretin gene result in accumulation of large amyloid deposits of misfolded transthyretin molecules most prominently in peripheral nerves and the heart. Patients with hATTR typically present with polyneuropathy or autonomic dysfunction followed by cardiomyopathy which, if untreated, is fatal within 5 to 10 years.

















