A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.

Glutamate dehydrogenase is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typically, the α-ketoglutarate to glutamate reaction does not occur in mammals, as glutamate dehydrogenase equilibrium favours the production of ammonia and α-ketoglutarate. Glutamate dehydrogenase also has a very low affinity for ammonia, and therefore toxic levels of ammonia would have to be present in the body for the reverse reaction to proceed. However, in brain, the NAD+/NADH ratio in brain mitochondria encourages oxidative deamination. In bacteria, the ammonia is assimilated to amino acids via glutamate and aminotransferases. In plants, the enzyme can work in either direction depending on environment and stress. Transgenic plants expressing microbial GLDHs are improved in tolerance to herbicide, water deficit, and pathogen infections. They are more nutritionally valuable.
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.

Rutin is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a flavonoid glycoside found in a wide variety of plants, including citrus.

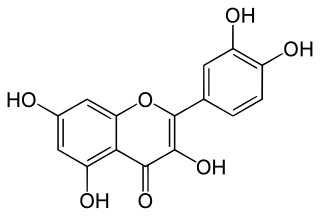
Quercitrin is a glycoside formed from the flavonoid quercetin and the deoxy sugar rhamnose.

Urocanase is the enzyme that catalyzes the second step in the degradation of histidine, the hydration of urocanate into imidazolonepropionate.
In enzymology, a succinylglutamate-semialdehyde dehydrogenase (EC 1.2.1.71) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-rhamnose 1-dehydrogenase (EC 1.1.1.173) is an enzyme that catalyzes the chemical reaction
In enzymology, a 5-carboxymethyl-2-hydroxymuconic-semialdehyde dehydrogenase (EC 1.2.1.60) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxymuconate-semialdehyde hydrolase (EC 3.7.1.9) is an enzyme that catalyzes the chemical reaction
The enzyme Lrhamnonate dehydratase (EC 4.2.1.90) catalyzes the chemical reaction
The enzyme L-rhamnono-1,4-lactonase (EC 3.1.1.65) catalyzes the reaction
In enzymology, a guanidinobutyrase (EC 3.5.3.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-succinylarginine dihydrolase (EC 3.5.3.23) is an enzyme that catalyzes the chemical reaction
In enzymology, a succinylglutamate desuccinylase (EC 3.5.1.96) is an enzyme that catalyzes the chemical reaction
3,4-Dehydroadipyl-CoA semialdehyde dehydrogenase (NADP+) (EC 1.2.1.77, BoxD, 3,4-dehydroadipyl-CoA semialdehyde dehydrogenase) is an enzyme with systematic name 3,4-didehydroadipyl-CoA semialdehyde:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction
Phenylacetyl-CoA 1,2-epoxidase (EC 1.14.13.149, ring 1,2-phenylacetyl-CoA epoxidase, phenylacetyl-CoA monooxygenase, PaaAC, PaaABC(D)E) is an enzyme with systematic name phenylacetyl-CoA:oxygen oxidoreductase (1,2-epoxidizing). This enzyme catalyses the following chemical reaction
trans-o-Hydroxybenzylidenepyruvate hydratase-aldolase (EC 4.1.2.45, 2′-hydroxybenzalpyruvate aldolase, NsaE, tHBPA hydratase-aldolase) is an enzyme with systematic name (3E)-4-(2-hydroxyphenyl)-2-oxobut-3-enoate hydro-lyase. This enzyme catalyses the following chemical reaction

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.







