 | |
 | |
| Names | |
|---|---|
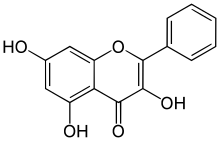
| IUPAC name 3,5,7-Trihydroxyflavone | |
| Systematic IUPAC name 3,5,7-Trihydroxy-2-phenyl-4H-1-benzopyran-4-one | |
| Other names Norizalpinin 3,5,7-triOH-Flavone | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.147 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240 g·mol−1 |
| Density | 1.579 g/mL |
| Melting point | 214 to 215 °C (417 to 419 °F; 487 to 488 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |