Contents
 | |
 | |
| Names | |
|---|---|
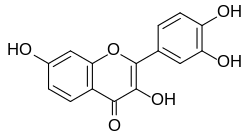
| IUPAC name 3,3′,4′,7-Tetrahydroxyflavone | |
| Systematic IUPAC name 2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-4H-1-benzopyran-4-one | |
| Other names 2-(3,4-Dihydroxyphenyl)-3,7-dihydroxychromen-4-one Cotinin (not to be confused with Cotinine) 5-Deoxyquercetin Superfustel Fisetholz Fietin Fustel Fustet Viset Junger fustik | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.669 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H10O6 | |
| Molar mass | 286.2363 g/mol |
| Density | 1.688 g/mL |
| Melting point | 330 °C (626 °F; 603 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Fisetin (7,3′,4′-flavon-3-ol) is a plant flavonol from the flavonoid group of polyphenols. [1] It occurs in many plants where it serves as a yellow pigment. It is found in many fruits and vegetables, such as strawberries, apples, persimmons, onions, and cucumbers. [2] [3] [4]
Its chemical formula was first described by Austrian chemist Josef Herzig in 1891. [5]