
The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.

Bacteriorhodopsin (Bop) is a protein used by Archaea, most notably by haloarchaea, a class of the Euryarchaeota. It acts as a proton pump; that is, it captures light energy and uses it to move protons across the membrane out of the cell. The resulting proton gradient is subsequently converted into chemical energy.

Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications.

Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making FRET extremely sensitive to small changes in distance.

Resonance Raman spectroscopy is a variant of Raman spectroscopy in which the incident photon energy is close in energy to an electronic transition of a compound or material under examination. This similarity in energy (resonance) leads to greatly increased intensity of the Raman scattering of certain vibrational modes, compared to ordinary Raman spectroscopy.

Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C16H10. This yellow-green solid is the smallest peri-fused PAH. Pyrene forms during incomplete combustion of organic compounds.

Avobenzone is an organic molecule and an oil-soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays.
Fluorescence correlation spectroscopy (FCS) is a statistical analysis, via time correlation, of stationary fluctuations of the fluorescence intensity. Its theoretical underpinning originated from L. Onsager's regression hypothesis. The analysis provides kinetic parameters of the physical processes underlying the fluctuations. One of the interesting applications of this is an analysis of the concentration fluctuations of fluorescent particles (molecules) in solution. In this application, the fluorescence emitted from a very tiny space in solution containing a small number of fluorescent particles (molecules) is observed. The fluorescence intensity is fluctuating due to Brownian motion of the particles. In other words, the number of the particles in the sub-space defined by the optical system is randomly changing around the average number. The analysis gives the average number of fluorescent particles and average diffusion time, when the particle is passing through the space. Eventually, both the concentration and size of the particle (molecule) are determined. Both parameters are important in biochemical research, biophysics, and chemistry.
A molecular logic gate is a molecule that performs a logical operation based on one or more physical or chemical inputs and a single output. The field has advanced from simple logic systems based on a single chemical or physical input to molecules capable of combinatorial and sequential operations such as arithmetic operations. Molecular logic gates work with input signals based on chemical processes and with output signals based on spectroscopic phenomena.

Flavonols are a class of flavonoids that have the 3-hydroxyflavone backbone. Their diversity stems from the different positions of the phenolic –OH groups. They are distinct from flavanols such as catechin, another class of flavonoids, and an unrelated group of metabolically important molecules, the flavins, derived from the yellow B vitamin riboflavin.
The Benesi–Hildebrand method is a mathematical approach used in physical chemistry for the determination of the equilibrium constant K and stoichiometry of non-bonding interactions. This method has been typically applied to reaction equilibria that form one-to-one complexes, such as charge-transfer complexes and host–guest molecular complexation.
A model lipid bilayer is any bilayer assembled in vitro, as opposed to the bilayer of natural cell membranes or covering various sub-cellular structures like the nucleus. They are used to study the fundamental properties of biological membranes in a simplified and well-controlled environment, and increasingly in bottom-up synthetic biology for the construction of artificial cells. A model bilayer can be made with either synthetic or natural lipids. The simplest model systems contain only a single pure synthetic lipid. More physiologically relevant model bilayers can be made with mixtures of several synthetic or natural lipids.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.

Mearnsetin is an O-methylated flavonol. It can be found in Eucalyptus globulus and in Elaeocarpus lanceofolius. The compound has antioxidative properties.

The term macromolecular assembly (MA) refers to massive chemical structures such as viruses and non-biologic nanoparticles, cellular organelles and membranes and ribosomes, etc. that are complex mixtures of polypeptide, polynucleotide, polysaccharide or other polymeric macromolecules. They are generally of more than one of these types, and the mixtures are defined spatially, and with regard to their underlying chemical composition and structure. Macromolecules are found in living and nonliving things, and are composed of many hundreds or thousands of atoms held together by covalent bonds; they are often characterized by repeating units. Assemblies of these can likewise be biologic or non-biologic, though the MA term is more commonly applied in biology, and the term supramolecular assembly is more often applied in non-biologic contexts. MAs of macromolecules are held in their defined forms by non-covalent intermolecular interactions, and can be in either non-repeating structures, or in repeating linear, circular, spiral, or other patterns. The process by which MAs are formed has been termed molecular self-assembly, a term especially applied in non-biologic contexts. A wide variety of physical/biophysical, chemical/biochemical, and computational methods exist for the study of MA; given the scale of MAs, efforts to elaborate their composition and structure and discern mechanisms underlying their functions are at the forefront of modern structure science.
YOYO-1 is a green fluorescent dye used in DNA staining. It belongs to the family of monomethine cyanine dyes and is a tetracationic homodimer of Oxazole Yellow, typically available as tetraiodide salt. In aqueous buffer, free YOYO-1 dye has very low fluorescence quantum yield. However, the intensity of fluorescence increases 3200 times upon binding through bis-intercalation to double-stranded DNA.
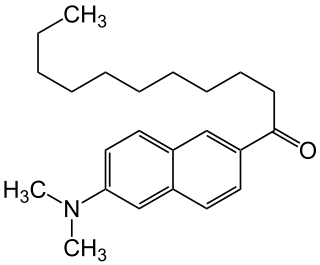
Laurdan is an organic compound which is used as a fluorescent dye when applied to fluorescence microscopy. It is used to investigate membrane qualities of the phospholipid bilayers of cell membranes. One of its most important characteristics is its sensitivity to membrane phase transitions as well as other alterations to membrane fluidity such as the penetration of water.
Excited state intramolecular proton transfer (ESIPT) is a process in which photoexcited molecules relax their energy through tautomerization by transfer of protons. Some kinds of molecules could have different minimum-energy tautomers in different electronic states, and if the molecular structure of minimum-energy tautomer in the excited state is proton-transferred geometry between neighboring atoms, proton transfer in excited state can occur. The tautomerization often takes the form of keto-enol tautomerism.

Dirubidium is a molecular substance containing two atoms of rubidium found in rubidium vapour. Dirubidium has two active valence electrons. It is studied both in theory and with experiment. The rubidium trimer has also been observed.
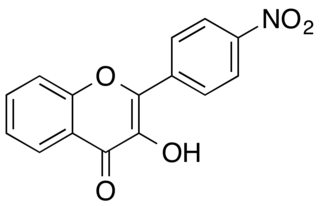
4'-Nitroflavonol is a pale yellow solid. This substance belongs to the subclass of flavonols of the class of flavonoids.















