
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus Vitis. Grapes are a non-climacteric type of fruit, generally occurring in clusters.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. As of 2012, there were between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.
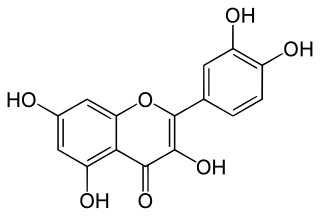
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.

The garden strawberry is a widely grown hybrid species of the genus Fragaria in the rose family, Rosaceae, collectively known as the strawberries, which are cultivated worldwide for their fruit. The fruit is widely appreciated for its characteristic aroma, bright red color, juicy texture, and sweetness. It is consumed in large quantities, either fresh or in such prepared foods as jam, juice, pies, ice cream, milkshakes, and chocolates. Artificial strawberry flavorings and aromas are also widely used in products such as confectionery, soap, lip gloss, perfume, and many others.
Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.
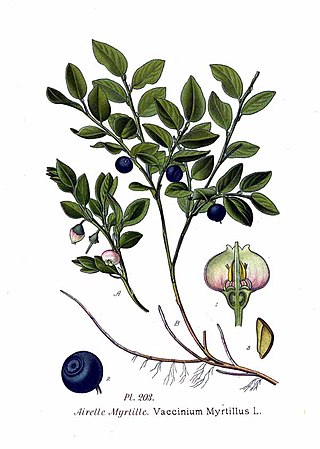
Vaccinium myrtillus or European blueberry is a holarctic species of shrub with edible fruit of blue color, known by the common names bilberry, blaeberry, wimberry, and whortleberry. It is more precisely called common bilberry or blue whortleberry to distinguish it from other Vaccinium relatives.

Paeonia lactiflora is a species of herbaceous perennial flowering plant in the family Paeoniaceae, native to central and eastern Asia from eastern Tibet across northern China to eastern Siberia.

Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a natural flavonol, a type of flavonoid, found in a variety of plants and plant-derived foods including kale, beans, tea, spinach, and broccoli. Kaempferol is a yellow crystalline solid with a melting point of 276–278 °C (529–532 °F). It is slightly soluble in water and highly soluble in hot ethanol, ethers, and DMSO. Kaempferol is named for 17th-century German naturalist Engelbert Kaempfer.

Peonidin is an O-methylated anthocyanidin derived from Cyanidin, and a primary plant pigment. Peonidin gives purplish-red hues to flowers such as the peony, from which it takes its name, and roses. It is also present in some blue flowers, such as the morning glory.

In enzymology, a flavonol 3-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart named a chemical compound that gives flowers a blue color, Anthokyan, in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

The color of wine is one of the most easily recognizable characteristics of wines. Color is also an element in wine tasting since heavy wines generally have a deeper color. The accessory traditionally used to judge the wine color was the tastevin, a shallow cup allowing one to see the color of the liquid in the dim light of a cellar. The color is an element in the classification of wines.

Huckleberry is a name used in North America for several plants in the family Ericaceae, in two closely related genera: Vaccinium and Gaylussacia.

Tricin is a chemical compound. It is an O-methylated flavone, a type of flavonoid. It can be found in rice bran and sugarcane.

Isorhamnetin is an O-methylated flavon-ol from the class of flavonoids. A common food source of this 3'-methoxylated derivative of quercetin and its glucoside conjugates are pungent yellow or red onions, in which it is a minor pigment, quercetin-3,4'-diglucoside and quercetin-4'-glucoside and the aglycone quercetin being the major pigments. Pears, olive oil, wine and tomato sauce are rich in isorhamnetin. Almond skin is a rich source of isorhamnetin-3-O-rutinoside and isorhamnetin-3-O-glucoside, in some cultivars they comprise 75% of the polyphenol content, the total of which can exceed 10 mg/100 gram almond. Others sources include the spice, herbal medicinal and psychoactive Mexican tarragon (Tagetes lucida), which is described as accumulating isorhamnetin and its 7-O-glucoside derivate. Nopal is also a good source of isorhamnetin, which can be extracted by supercritical fluid extraction assisted by enzymes.

Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols. It is extracted from plants such as Siberian larch and milk thistle.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

Laricitrin is an O-methylated flavonol, a type of flavonoid. It is found in red grape and in Vaccinium uliginosum. It is one of the phenolic compounds present in wine.

Ideain, the cyanidin 3-O-galactoside, is an anthocyanin, a type of plant pigment.



















