Enoyl-CoA-(∆) isomerase (EC 5.3.3.8, also known as dodecenoyl-CoA- isomerase, 3,2-trans-enoyl-CoA isomerase, ∆3 ,∆2 -enoyl-CoA isomerase, or acetylene-allene isomerase, is an enzyme that catalyzes the conversion of cis- or trans-double bonds of coenzyme A bound fatty acids at gamma-carbon to trans double bonds at beta-carbon as below:

Rutin is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a flavonoid glycoside found in a wide variety of plants, including citrus.

Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone is hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.

Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.

Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one).
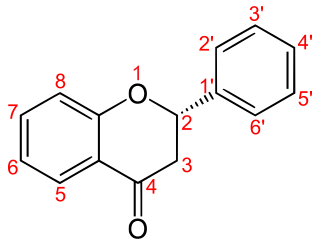
The flavanones, a type of flavonoids, are various aromatic, colorless ketones derived from flavone that often occur in plants as glycosides.

Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a class of organic compounds found mainly in plants as natural defense mechanisms and as synthetic intermediates. CHS was the first type III PKS to be discovered. It is the first committed enzyme in flavonoid biosynthesis. The enzyme catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone.
In enzymology, a 3-carboxy-cis,cis-muconate cycloisomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a carboxy-cis,cis-muconate cyclase is an enzyme that catalyzes the chemical reaction
In enzymology, a chloromuconate cycloisomerase is an enzyme that catalyzes the chemical reaction

Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.
In enzymology, a glucose-6-phosphate 1-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, a muconolactone Δ-isomerase is an enzyme that catalyzes the chemical reaction

In enzymology, a phosphoenolpyruvate mutase is an enzyme that catalyzes the chemical reaction
The enzyme indole-3-glycerol-phosphate synthase (IGPS) (EC 4.1.1.48) catalyzes the chemical reaction
The enzyme hydroperoxide dehydratase (EC 4.2.1.92) catalyzes the chemical reaction

Tricin is a chemical compound. It is an O-methylated flavone, a type of flavonoid. It can be found in rice bran and sugarcane.
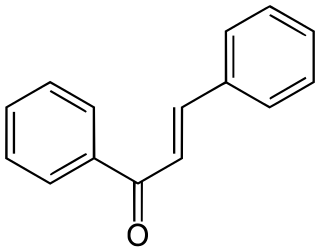
Chalconoids, also known as chalcones, are natural phenols derived from chalcone. They form the central core for a variety of important biological compounds.