
Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.

Benzylamine is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth.

Danishefsky's diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-buta-1,3-diene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The methoxy group promotes highly regioselective additions. The diene is known to react with amines, aldehydes, alkenes and alkynes. Reactions with imines and nitro-olefins have been reported.

Prostaglandin-I synthase also known as prostaglandin I2 (prostacyclin) synthase (PTGIS) or CYP8A1 is an enzyme involved in prostanoid biosynthesis that in humans is encoded by the PTGIS gene. This enzyme belongs to the family of cytochrome P450 isomerases.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.

In enzymology, a pyruvate synthase is an enzyme that catalyzes the interconversion of pyruvate and acetyl-CoA. It is also called pyruvate:ferredoxin oxidoreductase (PFOR).
In enzymology, an ent-copalyl diphosphate synthase is an enzyme that catalyzes the chemical reaction:
In enzymology, a 2-isopropylmalate synthase (EC 2.3.3.13) is an enzyme that catalyzes the chemical reaction
In enzymology, a cysteine synthase is an enzyme that catalyzes the chemical reaction

In enzymology, a sucrose synthase is an enzyme that catalyzes the chemical reaction
In organic chemistry, an intramolecular Diels-Alder cycloaddition is a Diels–Alder reaction in which the diene and a dienophile are both part of the same molecule. The reaction leads to the formation of the same cyclohexene-like structure as usual for a Diels–Alder reaction, but as part of a more complex fused or bridged cyclic ring system. This reaction gives rise to various natural derivatives of decalin.
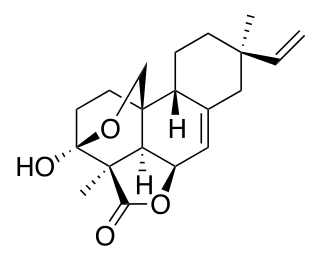
Momilactone B is an allelopathic agent produced from the roots of rice. It has been shown to be produced in high concentrations by the roots of rice seedlings. The production of momilactone B has also been induced in response to infection by blast fungus or irradiated with UV light. More recently it has been shown to be a potential chemotherapeutic agent against human colon cancer.

Macrophomic acid is a fungal metabolite isolated from the fungus Macrophoma commelinae. The enzyme macrophomate synthase converts 5-acetyl-4-methoxy-6-methyl-2-pyrone to 4-acetyl-3-methoxy-5-methyl-benzoic acid through an unusual intermolecular Diels-Alder reaction. The pathway to formation of macrophomic acid suggests that the enzyme is a natural Diels-Alderase. Formation of this type of aromatic ring compound normally proceeds via the shikimate and polyketide pathways; however, the production of macrophomic acid by macrophomate synthase proceeds totally differently. Learning about the production of macrophomic acid by a possible natural Diels-Alderase enzyme is important in understanding enzyme catalytic mechanisms. This knowledge can then be applied to organic synthesis.

Betaenone B, like other betaenones, is a secondary metabolite isolated from the fungus Pleospora betae, a plant pathogen. Its phytotoxic properties have been shown to cause sugar beet leaf spots, which is characterized by black, pycnidia containing, concentric circles eventually leading to necrosis of the leaf tissue. Of the seven phytotoxins isolated in fungal leaf spots from sugar beet, betaenone B showed the least amount of phytotoxicity showing only 8% inhibition of growth while betaenone A and C showed 73% and 89% growth inhibition, respectively. Betaenone B is therefore not considered toxic to the plant, but will produce leaf spots when present in high concentrations (0.33 μg/μL). While the mechanism of action of betaenone B has yet to be elucidated, betaenone C has been shown to inhibit RNA and protein synthesis. Most of the major work on betaenone B, including the initial structure elucidation of betaenone A, B and C as well as the partial elucidation mechanism of biosynthesis, was presented in three short papers published between 1983–88. The compounds were found to inhibit a variety of protein kinases signifying a possible role in cancer treatment.
Prosolanapyrone-II oxidase (EC 1.1.3.42, Sol5, SPS, solanapyrone synthase (bifunctional enzyme: prosolanapyrone II oxidase/prosolanapyrone III cycloisomerase), prosolanapyrone II oxidase) is an enzyme with systematic name prosolanapyrone-II:oxygen 3'-oxidoreductase. This enzyme catalyses the following chemical reaction
Fatty-acid peroxygenase is an enzyme with systematic name fatty acid:hydroperoxide oxidoreductase (RH-hydroxylating). This enzyme catalyses the following chemical reaction
3-hexulose-6-phosphate synthase is an enzyme with systematic name D-arabino-hex-3-ulose-6-phosphate formaldehyde-lyase (D-ribulose-5-phosphate-forming). This enzyme catalyses the following chemical reaction
β-amyrin synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction
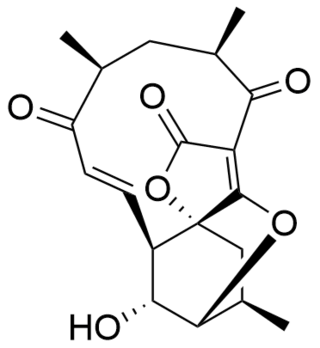
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C. Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB, thereby depleting the biosynthesis of p-aminobenzoate.
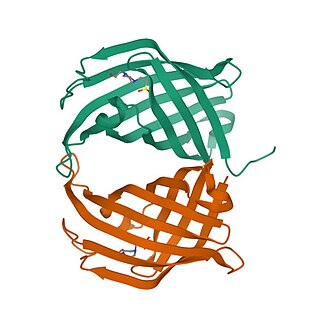
Spirotetronate cyclase AbyU is an enzyme responsible for catalyzing the Diels-Alder reaction in the abyssomicin C biosynthetic pathway. A key step in the biosynthesis of this compound catalyzed by AbyU involves intramolecular [4+2] cycloaddition—also known as the Diels-Alder reaction—to form a heterobicyclic ring system precursor consisting of tetronic acid and a cyclohexene ring that are spiro-linked.












