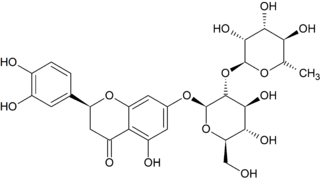A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 and thus with the empirical formula Cm(H2O)n, which does not mean the H has covalent bonds with O. However, not all carbohydrates conform to this precise stoichiometric definition, nor are all chemicals that do conform to this definition automatically classified as carbohydrates.

A disaccharide is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose.
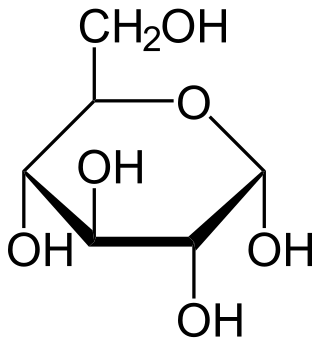
Glucose is a sugar with the molecular formula C6H12O6. Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world.
Monosaccharides, also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.

Maltose, also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar.

Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan. Since heparins depend on the activity of antithrombin, they are considered anticoagulants. Specifically it is also used in the treatment of heart attacks and unstable angina. It is given intravenously or by injection under the skin. Other uses for its anticoagulant properties include inside blood specimen test tubes and kidney dialysis machines.

Maltase is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall.

Trehalose is a sugar consisting of two molecules of glucose. It is also known as mycose or tremalose. Some bacteria, fungi, plants and invertebrate animals synthesize it as a source of energy, and to survive freezing and lack of water.
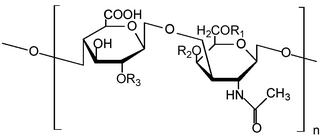
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.

Cellobiose is a disaccharide with the formula (C6H7(OH)4O)2O. It is classified as a reducing sugar. In terms of its chemical structure, it is derived from the condensation of a pair of β-glucose molecules forming a β(1→4) bond. It can be hydrolyzed to glucose enzymatically or with acid. Cellobiose has eight free alcohol (OH) groups, one acetal linkage and one hemiacetal linkage, which give rise to strong inter- and intramolecular hydrogen bonds. It is a white solid.

Isomalt is a sugar substitute, a mixture of the two disaccharide alcohols 1,6-GPS and 1,1-GPM. It is used primarily for its sugar-like physical properties. It has little to no impact on blood sugar levels, and does not stimulate the release of insulin. It also does not promote tooth decay and is considered to be tooth-friendly. Its energy value is 2 kcal per gram, half that of sugars. It is less sweet than sugar, but can be blended with high-intensity sweeteners such as sucralose to create a mixture with the same sweetness as sucrose (‘sugar’).

Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).

Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. It is in this form that HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB, and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.
The enzyme protein-glucosylgalactosylhydroxylysine glucosidase (EC 3.2.1.107) catalyzes the following chemical reaction:

Turanose is a reducing disaccharide. The d-isomer is naturally occurring. Its systematic name is α-d-glucopyranosyl-(1→3)-α-d-fructofuranose. It is an analog of sucrose not metabolized by higher plants, but rather acquired through the action of sucrose transporters for intracellular carbohydrate signaling. In addition to its involvement in signal transduction, d-(+)-turanose can also be used as a carbon source by many organisms including numerous species of bacteria and fungi.
Arabinogalactan, also known as galactoarabinan, larch arabinogalactan, and larch gum, is a biopolymer consisting of arabinose and galactose monosaccharides. Two classes of arabinogalactans are found in nature: plant arabinogalactan and microbial arabinogalactan. In plants, it is a major component of many gums, including gum arabic and gum ghatti. It is often found attached to proteins, and the resulting arabinogalactan protein (AGP) functions as both an intercellular signaling molecule and a glue to seal plant wounds.
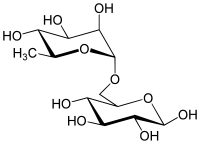
Isomaltooligosaccharide (IMO) is a mixture of short-chain carbohydrates which has a digestion-resistant property. IMO is found naturally in some foods, as well as being manufactured commercially. The raw material used for manufacturing IMO is starch, which is enzymatically converted into a mixture of isomaltooligosaccharides.
Rhamnogalacturonan exolyase is an enzyme with systematic name α-L-rhamnopyranosyl-(1→4)-α-D-galactopyranosyluronate exolyase. This enzyme catalyses the following chemical reaction
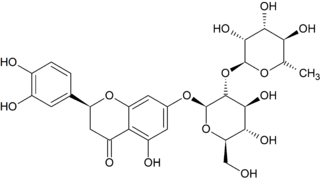
Neoeriocitrin is a 7-O-glycoside of the flavanone eriodictyol and the disaccharide neohesperidose . Note that the 'neo' in the name in this case does not refer to the position of the B-ring, but refer to the glycosyl moiety.