
A gamete is a haploid cell that fuses with another haploid cell during fertilization in organisms that reproduce sexually. Gametes are an organism's reproductive cells, also referred to as sex cells. The name gamete was introduced by the German cytologist Eduard Strasburger in 1878.

A spermatozoon is a motile sperm cell, or moving form of the haploid cell that is the male gamete. A spermatozoon joins an ovum to form a zygote.

Fertilisation or fertilization, also known as generative fertilisation, syngamy and impregnation, is the fusion of gametes to give rise to a zygote and initiate its development into a new individual organism or offspring. While processes such as insemination or pollination, which happen before the fusion of gametes, are also sometimes informally referred to as fertilisation, these are technically separate processes. The cycle of fertilisation and development of new individuals is called sexual reproduction. During double fertilisation in angiosperms, the haploid male gamete combines with two haploid polar nuclei to form a triploid primary endosperm nucleus by the process of vegetative fertilisation.

Intracytoplasmic sperm injection is an in vitro fertilization (IVF) procedure in which a single sperm cell is injected directly into the cytoplasm of an egg. This technique is used in order to prepare the gametes for the obtention of embryos that may be transferred to a maternal uterus. With this method, the acrosome reaction is skipped.

Gametogenesis is a biological process by which diploid or haploid precursor cells undergo cell division and differentiation to form mature haploid gametes. Depending on the biological life cycle of the organism, gametogenesis occurs by meiotic division of diploid gametocytes into various gametes, or by mitosis. For example, plants produce gametes through mitosis in gametophytes. The gametophytes grow from haploid spores after sporic meiosis. The existence of a multicellular, haploid phase in the life cycle between meiosis and gametogenesis is also referred to as alternation of generations.

A germ cell is any cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they undergo meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have germ cells designated in early development. Instead, germ cells can arise from somatic cells in the adult, such as the floral meristem of flowering plants.

For fertilization to happen between a sperm and egg cell, a sperm must first fuse with the plasma membrane and then penetrate the female egg cell to fertilize it. While the fusion of the sperm cell with the egg cell's plasma membrane is relatively straightforward, penetrating the egg's protective layers, such as the zona pellucida, presents a significant challenge. Therefore, sperm cells go through a process known as the acrosome reaction, which is the reaction that occurs in the acrosome of the sperm as it approaches the egg.

Spermatogenesis is the process by which haploid spermatozoa develop from germ cells in the seminiferous tubules of the testicle. This process starts with the mitotic division of the stem cells located close to the basement membrane of the tubules. These cells are called spermatogonial stem cells. The mitotic division of these produces two types of cells. Type A cells replenish the stem cells, and type B cells differentiate into primary spermatocytes. The primary spermatocyte divides meiotically into two secondary spermatocytes; each secondary spermatocyte divides into two equal haploid spermatids by Meiosis II. The spermatids are transformed into spermatozoa (sperm) by the process of spermiogenesis. These develop into mature spermatozoa, also known as sperm cells. Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa and four haploid cells.

The spermatid is the haploid male gametid that results from division of secondary spermatocytes. As a result of meiosis, each spermatid contains only half of the genetic material present in the original primary spermatocyte.
Capacitation is the penultimate step in the maturation of mammalian spermatozoa and is required to render them competent to fertilize an oocyte. This step is a biochemical event; the sperm move normally and look mature prior to capacitation. In vivo, capacitation occurs after ejaculation, when the spermatozoa leave the vagina and enter the upper female reproductive tract. The uterus aids in the steps of capacitation by secreting sterol-binding albumin, lipoproteins, and proteolytic and glycosidasic enzymes such as heparin.

Spermatocytes are a type of male gametocyte in animals. They derive from immature germ cells called spermatogonia. They are found in the testis, in a structure known as the seminiferous tubules. There are two types of spermatocytes, primary and secondary spermatocytes. Primary and secondary spermatocytes are formed through the process of spermatocytogenesis.
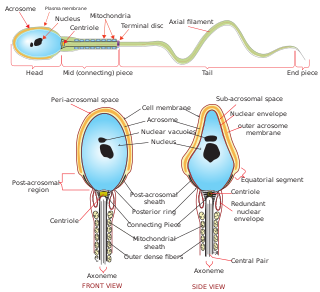
Sperm is the male reproductive cell, or gamete, in anisogamous forms of sexual reproduction. Animals produce motile sperm with a tail known as a flagellum, which are known as spermatozoa, while some red algae and fungi produce non-motile sperm cells, known as spermatia. Flowering plants contain non-motile sperm inside pollen, while some more basal plants like ferns and some gymnosperms have motile sperm.
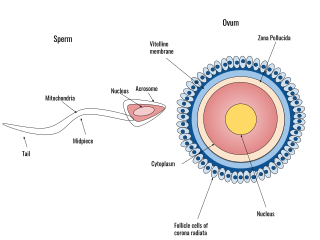
Human fertilization is the union of an egg and sperm, occurring primarily in the ampulla of the fallopian tube. The result of this union leads to the production of a fertilized egg called a zygote, initiating embryonic development. Scientists discovered the dynamics of human fertilization in the 19th century.
Male infertility refers to a sexually mature male's inability to impregnate a fertile female. In humans, it accounts for 40–50% of infertility. It affects approximately 7% of all men. Male infertility is commonly due to deficiencies in the semen, and semen quality is used as a surrogate measure of male fecundity. More recently, advance sperm analyses that examine intracellular sperm components are being developed.

Sperm motility describes the ability of sperm to move properly through the female reproductive tract or through water to reach the egg. Sperm motility can also be thought of as the quality, which is a factor in successful conception; sperm that do not "swim" properly will not reach the egg in order to fertilize it. Sperm motility in mammals also facilitates the passage of the sperm through the cumulus oophorus and the zona pellucida, which surround the mammalian oocyte.

VEZT is a gene located on chromosome 12 and encodes for the protein vezatin. Vezatin is a major component of the cadherin-catenin complex that is critical to the formation and maintenance of adherens junctions. The protein is expressed in most epithelial cells and is crucial to the formation of cell-cell contact junctions. Mutations of the gene can lead to upregulation or downregulation of the protein which can have detrimental effects on physiological systems, particularly those involved in development.
Spermatozoa develop in the seminiferous tubules of the testes. During their development, the spermatogonia proceed through meiosis to become spermatozoa. Many changes occur during this process: the DNA in nuclei becomes condensed; the acrosome develops as a structure close to the nucleus. The acrosome is derived from the Golgi apparatus and contains hydrolytic enzymes important for fusion of the spermatozoon with an egg cell. During spermiogenesis, the nucleus condenses and changes shape. Abnormal shape change is a feature of sperm in male infertility. The acroplaxome is a structure found between the acrosomal membrane and the nuclear membrane. The acroplaxome contains structural proteins including keratin 5, F-actin and profilin IV.
Oocyteactivation is a series of processes that occur in the oocyte during fertilization.

A spermatogonial stem cell (SSC), also known as a type A spermatogonium, is a spermatogonium that does not differentiate into a spermatocyte, a precursor of sperm cells. Instead, they continue dividing into other spermatogonia or remain dormant to maintain a reserve of spermatogonia. Type B spermatogonia, on the other hand, differentiate into spermatocytes, which in turn undergo meiosis to eventually form mature sperm cells.
Spermatogenesis-associated protein 16 is a mammalian protein encoded by the SPATA16 gene. SPATA16, also known as NYD-SP12, is a developmental protein that aids in differentiation of germ cells for spermatogenesis and participates in acrosome formation for appropriate sperm-egg fusion. SPATA16 is located on chromosome 3 at position 26.31 and is a member of the tetratricopeptide repeat-like superfamily, which facilitate interactions and assemblies between proteins and protein complexes.















