
Gametogenesis is a biological process by which diploid or haploid precursor cells undergo cell division and differentiation to form mature haploid gametes. Depending on the biological life cycle of the organism, gametogenesis occurs by meiotic division of diploid gametocytes into various gametes, or by mitosis. For example, plants produce gametes through mitosis in gametophytes. The gametophytes grow from haploid spores after sporic meiosis. The existence of a multicellular, haploid phase in the life cycle between meiosis and gametogenesis is also referred to as alternation of generations.

A germ cell is any cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they undergo meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have germ cells designated in early development. Instead, germ cells can arise from somatic cells in the adult, such as the floral meristem of flowering plants.

Spermatogenesis is the process by which haploid spermatozoa develop from germ cells in the seminiferous tubules of the testicle. This process starts with the mitotic division of the stem cells located close to the basement membrane of the tubules. These cells are called spermatogonial stem cells. The mitotic division of these produces two types of cells. Type A cells replenish the stem cells, and type B cells differentiate into primary spermatocytes. The primary spermatocyte divides meiotically into two secondary spermatocytes; each secondary spermatocyte divides into two equal haploid spermatids by Meiosis II. The spermatids are transformed into spermatozoa (sperm) by the process of spermiogenesis. These develop into mature spermatozoa, also known as sperm cells. Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa and four haploid cells.

Sertoli cells are a type of sustentacular "nurse" cell found in human testes which contribute to the process of spermatogenesis as a structural component of the seminiferous tubules. They are activated by follicle-stimulating hormone (FSH) secreted by the adenohypophysis and express FSH receptor on their membranes.

Spermatocytes are a type of male gametocyte in animals. They derive from immature germ cells called spermatogonia. They are found in the testis, in a structure known as the seminiferous tubules. There are two types of spermatocytes, primary and secondary spermatocytes. Primary and secondary spermatocytes are formed through the process of spermatocytogenesis.
Reproductive biology includes both sexual and asexual reproduction.

A spermatogonium is an undifferentiated male germ cell. Spermatogonia undergo spermatogenesis to form mature spermatozoa in the seminiferous tubules of the testicles.
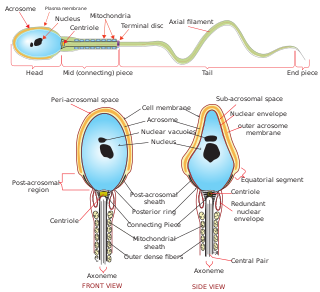
Sperm is the male reproductive cell, or gamete, in anisogamous forms of sexual reproduction. Animals produce motile sperm with a tail known as a flagellum, which are known as spermatozoa, while some red algae and fungi produce non-motile sperm cells, known as spermatia. Flowering plants contain non-motile sperm inside pollen, while some more basal plants like ferns and some gymnosperms have motile sperm.
Gametogonium are stem cells for gametes located within the gonads. They originate from primordial germ cells, which have migrated to the gonads. Male gametogonia which are located within the testes during development and adulthood are called spermatogonium. Female gametogonia, known as oogonium, are found within the ovaries of the developing foetus and were thought to be depleted at or after birth. Spermatogonia and oogonia are classified as sexually differentiated germ cells.
In developmental biology, the cells that give rise to the gametes are often set aside during embryonic cleavage. During development, these cells will differentiate into primordial germ cells, migrate to the location of the gonad, and form the germline of the animal.
Stem-cell niche refers to a microenvironment, within the specific anatomic location where stem cells are found, which interacts with stem cells to regulate cell fate. The word 'niche' can be in reference to the in vivo or in vitro stem-cell microenvironment. During embryonic development, various niche factors act on embryonic stem cells to alter gene expression, and induce their proliferation or differentiation for the development of the fetus. Within the human body, stem-cell niches maintain adult stem cells in a quiescent state, but after tissue injury, the surrounding micro-environment actively signals to stem cells to promote either self-renewal or differentiation to form new tissues. Several factors are important to regulate stem-cell characteristics within the niche: cell–cell interactions between stem cells, as well as interactions between stem cells and neighbouring differentiated cells, interactions between stem cells and adhesion molecules, extracellular matrix components, the oxygen tension, growth factors, cytokines, and the physicochemical nature of the environment including the pH, ionic strength and metabolites, like ATP, are also important. The stem cells and niche may induce each other during development and reciprocally signal to maintain each other during adulthood.

Sertoli cell-only syndrome (SCOS), also known as germ cell aplasia, is defined by azoospermia where the testicular seminiferous tubules are lined solely with sertoli cells. Sertoli cells contribute to the formation of the blood-testis barrier and aid in sperm generation. These cells respond to follicle-stimulating hormone, which is secreted by the hypothalamus and aids in spermatogenesis.

Calcium and integrin-binding protein 1 is a protein that in humans is encoded by the CIB1 gene and is located in Chromosome 15. The protein encoded by this gene is a member of the calcium-binding protein family. The specific function of this protein has not yet been determined; however this protein is known to interact with DNA-dependent protein kinase and may play a role in kinase-phosphatase regulation of DNA end-joining. This protein also interacts with integrin alpha(IIb)beta(3), which may implicate this protein as a regulatory molecule for alpha(IIb)beta(3).

Paired box protein Pax-7 is a protein that in humans is encoded by the PAX7 gene.
Gonocytes are the precursors of spermatogonia that differentiate in the testis from primordial germ cells around week 7 of embryonic development and exist up until the postnatal period, when they become spermatogonia. Despite some uses of the term to refer to the precursors of oogonia, it was generally restricted to male germ cells. Germ cells operate as vehicles of inheritance by transferring genetic and epigenetic information from one generation to the next. Male fertility is centered around continual spermatogonia which is dependent upon a high stem cell population. Thus, the function and quality of a differentiated sperm cell is dependent upon the capacity of its originating spermatogonial stem cell (SSC).

A spermatogonial stem cell (SSC), also known as a type A spermatogonium, is a spermatogonium that does not differentiate into a spermatocyte, a precursor of sperm cells. Instead, they continue dividing into other spermatogonia or remain dormant to maintain a reserve of spermatogonia. Type B spermatogonia, on the other hand, differentiate into spermatocytes, which in turn undergo meiosis to eventually form mature sperm cells.
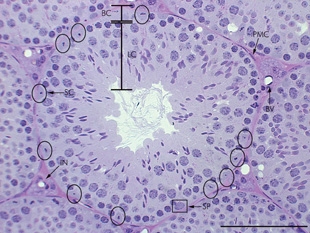
A peritubular myoid (PTM) cell is one of the smooth muscle cells which surround the seminiferous tubules in the testis. These cells are present in all mammals but their organization and abundance varies between species. The exact role of PTM cells is still somewhat uncertain and further work into this is needed. However, a number of functions of these cells have been established. They are contractile cells which contain actin filaments and are primarily involved in transport of spermatozoa through the tubules. They provide structural integrity to the tubules through their involvement in laying down the basement membrane. This has also been shown to affect Sertoli cell function and PTM cells also communicate with Sertoli cells through the secretion of growth factors and ECM components. Studies have shown PTM cells to be critical in achieving normal spermatogenesis. Overall, PTM cells have a role in both maintaining the structure of the tubules and regulating spermatogenesis through cellular interaction.
In vitro spermatogenesis is the process of creating male gametes (spermatozoa) outside of the body in a culture system. The process could be useful for fertility preservation, infertility treatment and may further develop the understanding of spermatogenesis at the cellular and molecular level.

The side effects of radiotherapy on fertility are a growing concern to patients undergoing radiotherapy as cancer treatments. Radiotherapy is essential for certain cancer treatments and often is the first point of call for patients. Radiation can be divided into two categories: ionising radiation (IR) and non-ionising radiation (NIR). IR is more dangerous than NIR and a source of this radiation is X-rays used in medical procedures, for example in radiotherapy.
The germ cell nest forms in the ovaries during their development. The nest consists of multiple interconnected oogonia formed by incomplete cell division. The interconnected oogonia are surrounded by somatic cells called granulosa cells. Later on in development, the germ cell nests break down through invasion of granulosa cells. The result is individual oogonia surrounded by a single layer of granulosa cells. There is also a comparative germ cell nest structure in the developing spermatogonia, with interconnected intracellular cytoplasmic bridges.











