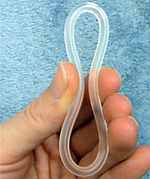
A hormonal intrauterine device (IUD), also known as a intrauterine system (IUS) with progestogen and sold under the brand name Mirena among others, is an intrauterine device that releases a progestogenic hormonal agent such as levonorgestrel into the uterus. It is used for birth control, heavy menstrual periods, and to prevent excessive build of the lining of the uterus in those on estrogen replacement therapy. It is one of the most effective forms of birth control with a one-year failure rate around 0.2%. The device is placed in the uterus and lasts three to eight years. Fertility often returns quickly following removal.
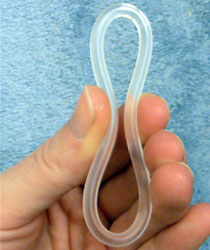
A contraceptive vaginal ring is a type of hormonal insert that is placed in the vagina for the purpose of birth control. The rings themselves utilize a plastic polymer matrix that is inlaid or embedded with contraceptive drug. This drug, often one or two hormones, is absorbed directly through the bloodstream through the cells that line the vaginal wall. Some vaginal rings contain both an estrogen and a progestin, which are available in Europe and the United States. Other vaginal rings contain just progesterone. The progesterone-only ring is only available in Latin America, exclusively for postpartum breastfeeding parents, therefore, it is not available in the United States.

Sulfadiazine is an antibiotic. Used together with pyrimethamine, a dihydrofolate reductase inhibitor, it is the treatment of choice for toxoplasmosis, which is caused by a protozoan parasite. It is a second-line treatment for otitis media, prophylaxis of rheumatic fever, chancroid, chlamydia, and infections by Haemophilus influenzae. It is also used as adjunct therapy for chloroquine-resistant malaria and several forms of bacterial meningitis. It is taken by mouth. Sulfadiazine is available in multiple generic tablets of 500 mg. For urinary tract infections, the usual dose is 4 to 6 grams daily in 3 to 6 divided doses.

Meglumine antimoniate is a medicine used to treat leishmaniasis. This includes visceral, mucocutaneous, and cutaneous leishmaniasis. It is given by injection into a muscle or into the area infected.
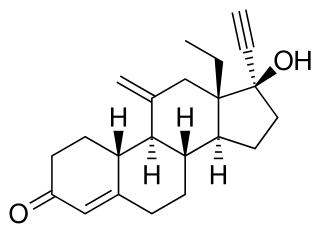
Etonogestrel is a medication which is used as a means of birth control for women. It is available as an implant placed under the skin of the upper arm under the brand names Nexplanon and Implanon, and in combination with ethinylestradiol, an estrogen, as a vaginal ring under the brand names NuvaRing and Circlet. Etonogestrel is effective as a means of birth control and lasts at least three or four years with some data showing effectiveness for five years. Following removal, fertility quickly returns.

Abacavir/lamivudine, sold under the brand name Kivexa among others, is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains abacavir and lamivudine. It is generally recommended for use with other antiretrovirals. It is commonly used as part of the preferred treatment in children. It is taken by mouth as a tablet.
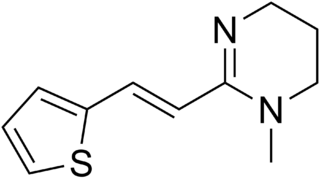
Pyrantel is a medication used to treat a number of parasitic worm infections. This includes ascariasis, hookworm infections, enterobiasis, trichostrongyliasis, and trichinellosis. It is taken by mouth.

Tick-borne encephalitis vaccine is a vaccine used to prevent tick-borne encephalitis (TBE). The disease is most common in Central and Eastern Europe, and Northern Asia. More than 87% of people who receive the vaccine develop immunity. It is not useful following the bite of an infected tick. It is given by injection into a muscle.

Ulipristal acetate, sold under the brand name Ella among others, is a medication used for emergency contraception and uterine fibroids. As emergency contraception it should be used within 120 hours of vaginally penetrating intercourse. For fibroids it may be taken for up to six months. It is taken by mouth.

Urea, also known as carbamide-containing cream, is used as a medication and applied to the skin to treat dryness and itching such as may occur in psoriasis, dermatitis, or ichthyosis. It may also be used to soften nails.
Sulfadoxine/pyrimethamine, sold under the brand name Fansidar, is a combination medication used to treat malaria. It contains sulfadoxine and pyrimethamine. For the treatment of malaria it is typically used along with other antimalarial medication such as artesunate. In areas of Africa with moderate to high rates of malaria, three doses are recommended during the second and third trimester of pregnancy.

Dasabuvir, sold under the brand name Exviera, is an antiviral medication for the treatment of hepatitis C. It is often used together with the combination medication ombitasvir/paritaprevir/ritonavir specifically for hepatitis C virus (HCV) type 1. Ribavirin may also additionally be used. These combinations result in a cure in more than 90% of people. It is taken by mouth.

Ethinylestradiol/levonorgestrel (EE/LNG) is a combined birth control pill made up of ethinylestradiol, an estrogen and levonorgestrel a progestin. It is used for birth control, symptoms of menstruation, endometriosis, and as emergency contraception. It is taken by mouth. Some preparations of EE/LNG additionally contain an iron supplement in the form of ferrous bisglycinate or ferrous fumarate.
Lamivudine/nevirapine/zidovudine (3TC/NVP/AZT) is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains lamivudine, nevirapine, and zidovudine. It is either used by itself or along with other antiretrovirals. It is a recommended treatment in those who are pregnant. It is taken by mouth twice a day.

Estradiol cypionate/medroxyprogesterone acetate (EC/MPA), sold under the brand name Cyclofem among others, is a form of combined injectable birth control. It contains estradiol cypionate (EC), an estrogen, and medroxyprogesterone acetate (MPA), a progestin. It is recommended for short-term use and is given once a month by injection into a muscle.
Dextran 70 is a type of fluid given by injection into a vein to expand blood volume. Specifically it is used for shock such as that caused by bleeding or burns when blood transfusions are not quickly available. However, it does not carry oxygen.

Zinc sulfate is used medically as a dietary supplement. Specifically it is used to treat zinc deficiency and to prevent the condition in those at high risk. This includes use together with oral rehydration therapy for children who have diarrhea. General use is not recommended. It may be taken by mouth or by injection into a vein.

Combined hormonal contraception (CHC), or combined birth control, is a form of hormonal contraception which combines both an estrogen and a progestogen in varying formulations.
Lisinopril/amlodipine, sold under the brand name Lisonorm among others, is a medication used to treat high blood pressure. It is a combination of lisinopril an ACE inhibitor with amlodipine a calcium channel blocker. It may be used when blood pressure is not well controlled with each of the two agents alone. It is taken by mouth.