Related Research Articles
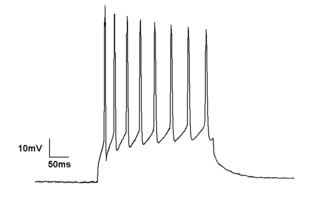
Electrophysiology is the branch of physiology that studies the electrical properties of biological cells and tissues. It involves measurements of voltage changes or electric current or manipulations on a wide variety of scales from single ion channel proteins to whole organs like the heart. In neuroscience, it includes measurements of the electrical activity of neurons, and, in particular, action potential activity. Recordings of large-scale electric signals from the nervous system, such as electroencephalography, may also be referred to as electrophysiological recordings. They are useful for electrodiagnosis and monitoring.

An action potential occurs when the membrane potential of a specific cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of excitable cells, which include animal cells like neurons and muscle cells, as well as some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.

The contraction of cardiac muscle in all animals is initiated by electrical impulses known as action potentials that in the heart are known as cardiac action potentials. The rate at which these impulses fire controls the rate of cardiac contraction, that is, the heart rate. The cells that create these rhythmic impulses, setting the pace for blood pumping, are called pacemaker cells, and they directly control the heart rate. They make up the cardiac pacemaker, that is, the natural pacemaker of the heart. In most humans, the highest concentration of pacemaker cells is in the sinoatrial (SA) node, the natural and primary pacemaker, and the resultant rhythm is a sinus rhythm.

Membrane potential is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges to move from the internal to exterior cellular environments and vice versa, as long as there is no acquisition of kinetic energy or the production of radiation. The concentration gradients of the charges directly determine this energy requirement. For the exterior of the cell, typical values of membrane potential, normally given in units of milli volts and denoted as mV, range from –80 mV to –40 mV.

In electrophysiology, the threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential. In neuroscience, threshold potentials are necessary to regulate and propagate signaling in both the central nervous system (CNS) and the peripheral nervous system (PNS).

The voltage clamp is an experimental method used by electrophysiologists to measure the ion currents through the membranes of excitable cells, such as neurons, while holding the membrane voltage at a set level. A basic voltage clamp will iteratively measure the membrane potential, and then change the membrane potential (voltage) to a desired value by adding the necessary current. This "clamps" the cell membrane at a desired constant voltage, allowing the voltage clamp to record what currents are delivered. Because the currents applied to the cell must be equal to the current going across the cell membrane at the set voltage, the recorded currents indicate how the cell reacts to changes in membrane potential. Cell membranes of excitable cells contain many different kinds of ion channels, some of which are voltage-gated. The voltage clamp allows the membrane voltage to be manipulated independently of the ionic currents, allowing the current–voltage relationships of membrane channels to be studied.

The patch clamp technique is a laboratory technique in electrophysiology used to study ionic currents in individual isolated living cells, tissue sections, or patches of cell membrane. The technique is especially useful in the study of excitable cells such as neurons, cardiomyocytes, muscle fibers, and pancreatic beta cells, and can also be applied to the study of bacterial ion channels in specially prepared giant spheroplasts.
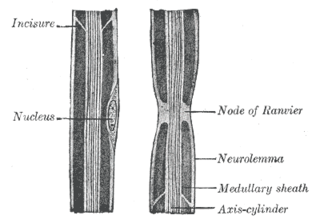
In neuroscience and anatomy, nodes of Ranvier, also known as myelin-sheath gaps, occur along a myelinated axon where the axolemma is exposed to the extracellular space. Nodes of Ranvier are uninsulated and highly enriched in ion channels, allowing them to participate in the exchange of ions required to regenerate the action potential. Nerve conduction in myelinated axons is referred to as saltatory conduction due to the manner in which the action potential seems to "jump" from one node to the next along the axon. This results in faster conduction of the action potential.

Electromyography (EMG) is a technique for evaluating and recording the electrical activity produced by skeletal muscles. EMG is performed using an instrument called an electromyograph to produce a record called an electromyogram. An electromyograph detects the electric potential generated by muscle cells when these cells are electrically or neurologically activated. The signals can be analyzed to detect abnormalities, activation level, or recruitment order, or to analyze the biomechanics of human or animal movement. Needle EMG is an electrodiagnostic medicine technique commonly used by neurologists. Surface EMG is a non-medical procedure used to assess muscle activation by several professionals, including physiotherapists, kinesiologists and biomedical engineers. In computer science, EMG is also used as middleware in gesture recognition towards allowing the input of physical action to a computer as a form of human-computer interaction.
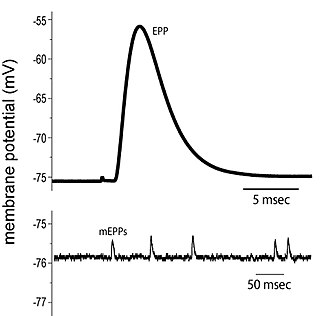
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.
Neural engineering is a discipline within biomedical engineering that uses engineering techniques to understand, repair, replace, or enhance neural systems. Neural engineers are uniquely qualified to solve design problems at the interface of living neural tissue and non-living constructs.
In neuroscience, single-unit recordings provide a method of measuring the electro-physiological responses of a single neuron using a microelectrode system. When a neuron generates an action potential, the signal propagates down the neuron as a current which flows in and out of the cell through excitable membrane regions in the soma and axon. A microelectrode is inserted into the brain, where it can record the rate of change in voltage with respect to time. These microelectrodes must be fine-tipped, impedance matching; they are primarily glass micro-pipettes, metal microelectrodes made of platinum, tungsten, iridium or even iridium oxide. Microelectrodes can be carefully placed close to the cell membrane, allowing the ability to record extracellularly.
Cardioplegia is a solution given to the heart during cardiac surgery, to minimize the damage caused by myocardial ischemia while the heart is paused.

Chronaxie is the minimum time required for an electric current double the strength of the rheobase to stimulate a muscle or a neuron. Rheobase is the lowest intensity with indefinite pulse duration which just stimulated muscles or nerves. Chronaxie is dependent on the density of voltage-gated sodium channels in the cell, which affect that cell's excitability. Chronaxie varies across different types of tissue: fast-twitch muscles have a lower chronaxie, slow-twitch muscles have a higher one. Chronaxie is the tissue-excitability parameter that permits choice of the optimum stimulus pulse duration for stimulation of any excitable tissue. Chronaxie (c) is the Lapicque descriptor of the stimulus pulse duration for a current of twice rheobasic (b) strength, which is the threshold current for an infinitely long-duration stimulus pulse. Lapicque showed that these two quantities (c,b) define the strength-duration curve for current: I = b(1+c/d), where d is the pulse duration. However, there are two other electrical parameters used to describe a stimulus: energy and charge. The minimum energy occurs with a pulse duration equal to chronaxie. Minimum charge (bc) occurs with an infinitely short-duration pulse. Choice of a pulse duration equal to 10c requires a current of only 10% above rheobase (b). Choice of a pulse duration of 0.1c requires a charge of 10% above the minimum charge (bc).

An Ussing chamber is an apparatus for measuring epithelial membrane properties. It can detect and quantify transport and barrier functions of living tissue. The Ussing chamber was invented by the Danish zoologist and physiologist Hans Henriksen Ussing in 1946.
In neurophysiology, several mathematical models of the action potential have been developed, which fall into two basic types. The first type seeks to model the experimental data quantitatively, i.e., to reproduce the measurements of current and voltage exactly. The renowned Hodgkin–Huxley model of the axon from the Loligo squid exemplifies such models. Although qualitatively correct, the H-H model does not describe every type of excitable membrane accurately, since it considers only two ions, each with only one type of voltage-sensitive channel. However, other ions such as calcium may be important and there is a great diversity of channels for all ions. As an example, the cardiac action potential illustrates how differently shaped action potentials can be generated on membranes with voltage-sensitive calcium channels and different types of sodium/potassium channels. The second type of mathematical model is a simplification of the first type; the goal is not to reproduce the experimental data, but to understand qualitatively the role of action potentials in neural circuits. For such a purpose, detailed physiological models may be unnecessarily complicated and may obscure the "forest for the trees". The FitzHugh–Nagumo model is typical of this class, which is often studied for its entrainment behavior. Entrainment is commonly observed in nature, for example in the synchronized lighting of fireflies, which is coordinated by a burst of action potentials; entrainment can also be observed in individual neurons. Both types of models may be used to understand the behavior of small biological neural networks, such as the central pattern generators responsible for some automatic reflex actions. Such networks can generate a complex temporal pattern of action potentials that is used to coordinate muscular contractions, such as those involved in breathing or fast swimming to escape a predator.

In neurophysiology, a dendritic spike refers to an action potential generated in the dendrite of a neuron. Dendrites are branched extensions of a neuron. They receive electrical signals emitted from projecting neurons and transfer these signals to the cell body, or soma. Dendritic signaling has traditionally been viewed as a passive mode of electrical signaling. Unlike its axon counterpart which can generate signals through action potentials, dendrites were believed to only have the ability to propagate electrical signals by physical means: changes in conductance, length, cross sectional area, etc. However, the existence of dendritic spikes was proposed and demonstrated by W. Alden Spencer, Eric Kandel, Rodolfo Llinás and coworkers in the 1960s and a large body of evidence now makes it clear that dendrites are active neuronal structures. Dendrites contain voltage-gated ion channels giving them the ability to generate action potentials. Dendritic spikes have been recorded in numerous types of neurons in the brain and are thought to have great implications in neuronal communication, memory, and learning. They are one of the major factors in long-term potentiation.
Electrical impedance myography, or EIM, is a non-invasive technique for the assessment of muscle health that is based on the measurement of the electrical impedance characteristics of individual muscles or groups of muscles. The technique has been used for the purpose of evaluating neuromuscular diseases both for their diagnosis and for their ongoing assessment of progression or with therapeutic intervention. Muscle composition and microscopic structure change with disease, and EIM measures alterations in impedance that occur as a result of disease pathology. EIM has been specifically recognized for its potential as an ALS biomarker by Prize4Life, a 501(c)(3) nonprofit organization dedicated to accelerating the discovery of treatments and cures for ALS. The $1M ALS Biomarker Challenge focused on identifying a biomarker precise and reliable enough to cut Phase II drug trials in half. The prize was awarded to Dr. Seward Rutkove, chief, Division of Neuromuscular Disease, in the Department of Neurology at Beth Israel Deaconess Medical Center and Professor of Neurology at Harvard Medical School, for his work in developing the technique of EIM and its specific application to ALS. It is hoped that EIM as a biomarker will result in the more rapid and efficient identification of new treatments for ALS. EIM has shown sensitivity to disease status in a variety of neuromuscular conditions, including radiculopathy, inflammatory myopathy, Duchenne muscular dystrophy, and spinal muscular atrophy.
Electrocochleography is a technique of recording electrical potentials generated in the inner ear and auditory nerve in response to sound stimulation, using an electrode placed in the ear canal or tympanic membrane. The test is performed by an otologist or audiologist with specialized training, and is used for detection of elevated inner ear pressure or for the testing and monitoring of inner ear and auditory nerve function during surgery.
John Walter Woodbury (1923–2017) was an American electrophysiologist and author of the first textbook explanation of the Hodgkin-Huxley_model studies of the action potential. He applied physical and mathematical techniques to experimentally elucidate the nature of electrical excitability in cells. He was also involved in the experimental and theoretical investigations of the mechanisms of ion penetration through the ion channels in muscle membranes, the regulation of cellular acid-base balance and the control of epileptic seizures by repetitive Vagus nerve stimulation.
References
- ↑ Stämpfli, R (1954). "A new method for measuring membrane potentials with external electrodes". Experientia. 10 (12): 508–509. doi:10.1007/BF02166189. PMID 14353097. S2CID 41384989.
- ↑ Pooler, JP; Valenzeno, DP. (1983). "Reexamination of the double sucrose gap technique for the study of lobster giant axons. Theory and experiments". Biophys J. 44 (2): 261–269. Bibcode:1983BpJ....44..261P. doi:10.1016/S0006-3495(83)84298-2. PMC 1434829 . PMID 6652217.
- 1 2 Akert, K. (August 1996). Swiss Contributions to the Neurosciences in Four Hundred Years: From the Renaissance to the Present. Verlag der Fachvereine Hochschulverlag AG an der ETH Zurich. ISBN 978-3728123626.
- ↑ Bennett, Max R (2001). "Discovery of the electrical signs of inhibitory transmission: the inhibitory junction potential". History of the Synapse. Amsterdam: OPA. pp. 114–118. ISBN 978-9058232335.
- ↑ Stämpfli, Robert (1988). "This Week's Citation Classic: Stämpfli, R. A new method for measuring membrane potentials with external electrodes" (PDF). Current Contents. 31 (34): 19.
- ↑ Leoty, C; Alix, J. (1976). "Some Technical Improvements for the Voltage Clamp with the Double Sucrose Gap: Pflügers Archiv". European Journal of Physiology. 365 (1): 95–97. doi:10.1007/BF00583633. PMID 988547. S2CID 20867630.
- 1 2 3 4 5 6 Mert, T (2007). "Sucrose-Gap Technique: Advantages and Limitations". Neurophysiology. 38 (3): 237–241. doi:10.1007/s11062-007-0031-8. S2CID 35099707.
- 1 2 3 4 Gaginella, Timothy S (1996). Handbook of Methods in Gastrointestinal Pharmacology. Boca Raton, Florida: CRC Press, Inc. pp. 248–251. ISBN 978-0849383045.
- ↑ Moore, John W. (2007). "Voltage Clamp". Scholarpedia. 2 (9): 3060. Bibcode:2007SchpJ...2.3060M. doi: 10.4249/scholarpedia.3060 .
- ↑ Kosterlitz, H. W; Lees, G. M; Wallis., D. I (1968). "Resting and Action Potentials Recorded by the Sucrose-Gap Method in the Superior Cervical Ganglion of the Rabbit". J. Physiol. 195 (1): 39–53. doi:10.1113/jphysiol.1968.sp008445. PMC 1557902 . PMID 5639803.
- 1 2 3 Harrington, L; Johnson, EA (1973). "Voltage Clamp of Cardiac Muscle in a Double Sucrose GapA Feasibility Study". Biophysical Journal. 13 (7): 626–47. Bibcode:1973BpJ....13..626H. doi:10.1016/S0006-3495(73)86013-8. PMC 1484318 . PMID 4715582.
- ↑ Goldman, Yale; Martin, Morad (1977). "Measurement of Transmembrane Potential and Current in Cardiac Muscle: A New Voltage Clamp Method". J. Physiol. 268 (3): 613–54. doi:10.1113/jphysiol.1977.sp011875. PMC 1283682 . PMID 301933.
- ↑ McGuigan, John; Tsien, RW (1974). "Some Limitations of the Double Sucrose Gap, and Its Use in a Study of the Slow Outward Current in Mammalian Ventricular Muscle". J. Physiol. 240 (3): 775–806. doi:10.1113/jphysiol.1974.sp010634. PMC 1331006 . PMID 4415829.