Lq2 is a component of the venom of the scorpion Leiurus quinquestriatus . It blocks various potassium channels, among others the inward-rectifier potassium ion channel ROMK1. [1]
Contents

Lq2 is a component of the venom of the scorpion Leiurus quinquestriatus . It blocks various potassium channels, among others the inward-rectifier potassium ion channel ROMK1. [1]

Lq2 is also known as Potassium channel toxin alpha-KTx 1.2, Charybdotoxin-2, ChTX-Lq2, ChTx-d, Toxin 18-2 or Lqh 18-2.
The name Lq2 refers to the name of the animal species in which the toxin can be found. [2] Lq2 can be found in the scorpion Leiurus quinquestriatus (Lq). [3] [4] Lq2 is structurally similar to Lq1, which had been found previously and which is also a potassium channel blocker.
Lq2 is a component of the venom of the scorpion Leiurus quinquestriatus, known under various names, for example the deathstalker, the Israeli desert scorpion or the yellow scorpion.
Lq2 is a small peptide of 37 amino acids. Lq2 contains the classical scorpion toxin alpha-beta scaffold and is structurally similar to the neurotoxin Charybdotoxin (CTX). [5] Lq2 consists of an α-helix and a β-sheet, connected by an αβ3 loop containing disulfide bridges. The proteins three-dimensional structure has been reconstructed using nuclear magnetic resonance techniques. [5]
Lq2 interacts with all three types of potassium channels: [6] the voltage-activated, the Ca2+- activated and the inward-rectifier potassium channels. [7] The unique trait of Lq2 is its high affinity for certain inward-rectifier potassium ion channels, especially the Renal Outer Medullary Potassium channel ROMK1. This ion channel contributes to the regulation of the resting membrane potential.
Since all potassium channels share the same ion conducting outer pore structure, Lq2 binds to all three potassium channel types. Lq2 interacts with the T-X-X-T-X-GT-X-X-T-X-GY/F-Gt K+-selective section within the pore-forming region (P-region) of the ROMK1 ion channel. It blocks the channel, binding in a 1:1 stoichiometric ratio with its β-sheet. [8]
Potential use of Lq2 is mainly in cardiovascular diseases. [3]

Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

Charybdotoxin (ChTX) is a 37 amino acid neurotoxin from the venom of the scorpion Leiurus quinquestriatus hebraeus (deathstalker) that blocks calcium-activated potassium channels. This blockade causes hyperexcitability of the nervous system. It is a close homologue of agitoxin and both toxins come from Leiurus quinquestriatus hebraeus. It is named after Charybdis, a sea monster from Greek myth.
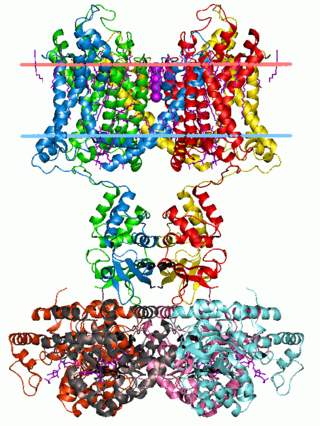
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.

The deathstalker is a species of scorpion, a member of the family Buthidae. It is also known as the Palestine yellow scorpion, Omdurman scorpion, and Naqab desert scorpion, as well as by many other colloquial names, which generally originate from the commercial captive trade of the animal. To eliminate confusion, especially important with potentially dangerous species, the scientific name is normally used to refer to them. The name Leiurus quinquestriatus roughly translates into English as "five-striped smooth-tail". In 2014, the subspecies L. q. hebraeus was separated from it and elevated to its own species Leiurus hebraeus. Other species of the genus Leiurus are also often referred to as "deathstalkers". Leiurus quinquestriatus is yellow, and 30–77 millimetres (1.2–3.0 in) long, with an average of 58 mm (2.3 in).
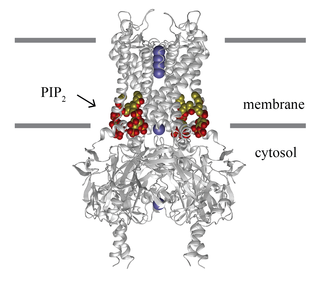
Inward-rectifier potassium channels (Kir, IRK) are a specific lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian and Alan Hodgkin in 1970 in skeletal muscle cells.
Tityustoxin is a toxin found in the venom of scorpions from the subfamily Tityinae. By binding to voltage-dependent sodium ion channels and potassium channels, they cause sialorrhea, lacrimation and rhinorrhea.

Scyllatoxin (also leiurotoxin I) is a toxin, from the scorpion Leiurus quinquestriatus hebraeus, which blocks small-conductance Ca2+-activated K+ channels. It is named after Scylla, a sea monster from Greek mythology. Charybdotoxin is also found in the venom from the same species of scorpion, and is named after the sea monster Charybdis. In Greek mythology, Scylla and Charybdis lived on rocks on opposing sides of a narrow strait of water.
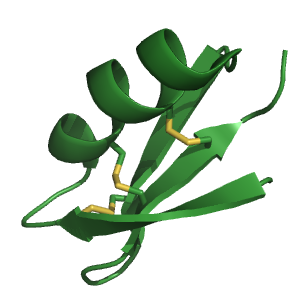
Agitoxin is a toxin found in the venom of the scorpion Leiurus quinquestriatus hebraeus. Other toxins found in this species include charybdotoxin (CTX). CTX is a close homologue of Agitoxin.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.
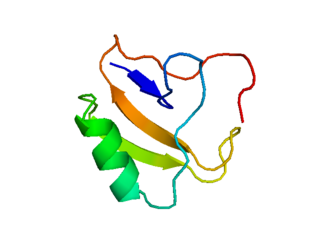
Scorpion toxins are proteins found in the venom of scorpions. Their toxic effect may be mammal- or insect-specific and acts by binding with varying degrees of specificity to members of the Voltage-gated ion channel superfamily; specifically, voltage-gated sodium channels, voltage-gated potassium channels, and Transient Receptor Potential (TRP) channels. The result of this action is to activate or inhibit the action of these channels in the nervous and cardiac organ systems. For instance, α-scorpion toxins MeuNaTxα-12 and MeuNaTxα-13 from Mesobuthus eupeus are neurotoxins that target voltage-gated Na+ channels (Navs), inhibiting fast inactivation. In vivo assays of MeuNaTxα-12 and MeuNaTxα-13 effects on mammalian and insect Navs show differential potency. These recombinants exhibit their preferential affinity for mammalian and insect Na+ channels at the α-like toxins' active site, site 3, in order to inactivate the cell membrane depolarization faster[6]. The varying sensitivity of different Navs to MeuNaTxα-12 and MeuNaTxα-13 may be dependent on the substitution of a conserved Valine residue for a Phenylalanine residue at position 1630 of the LD4:S3-S4 subunit or due to various changes in residues in the LD4:S5-S6 subunit of the Navs. Ultimately, these actions can serve the purpose of warding off predators by causing pain or to subdue predators.
Potassium channel blockers are agents which interfere with conduction through potassium channels.
Kaliotoxin (KTX) inhibits potassium flux through the Kv1.3 voltage-gated potassium channel and calcium-activated potassium channels by physically blocking the channel-entrance and inducing a conformational change in the K+-selectivity filter of the channel.
Hanatoxin is a toxin found in the venom of the Grammostola spatulata tarantula. The toxin is mostly known for inhibiting the activation of voltage-gated potassium channels, most specifically Kv4.2 and Kv2.1, by raising its activation threshold.
Tamulotoxin is a venomous neurotoxin from the Indian Red Scorpion.
Pi4 is a short toxin from the scorpion Pandinus imperator that blocks specific potassium channels.

Noxiustoxin (NTX) is a toxin from the venom of the Mexican scorpion Centruroides noxius Hoffmann which block voltage-dependent potassium channels and calcium-activated potassium channels.
ImKTx88 is a selective inhibitor of the Kv1 ion channel family that can be isolated from the venom of the Isometrus maculatus. This peptide belongs to the α-KTx subfamily and is classified as a pore-blocking toxin.
OdK2 is a toxin found in the venom of the Iranian scorpion Odonthobuthus doriae. It belongs to the α-KTx family, and selectively blocks the voltage-gated potassium channel Kv1.3 (KCNA3).
BmKTX is a scorpion neurotoxin which blocks the voltage gated potassium channel Kv1.3.
Alpha-Insect Toxin LqhαIT is a neurotoxic protein found in the venom of the Leiurus hebraeus, commonly known as the Hebrew deathstalker scorpion. It is classified as an alpha-toxin due to its effect on insect voltage-gated sodium channels, causing prolonged neuronal firing that leads to paralysis in affected insects. This toxin has been widely studied for its unique interaction with insect nervous systems and has potential applications in neurophysiological research.