Related Research Articles

Staphylococcus aureus is a gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe, meaning that it can grow without oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA). The bacterium is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.

An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.

An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. They can be chromosomally or plasmid encoded. They are heat labile, of low molecular weight and water-soluble. Enterotoxins are frequently cytotoxic and kill cells by altering the apical membrane permeability of the mucosal (epithelial) cells of the intestinal wall. They are mostly pore-forming toxins, secreted by bacteria, that assemble to form pores in cell membranes. This causes the cells to die.

Staphylococcus epidermidis is a Gram-positive bacterium, and one of over 40 species belonging to the genus Staphylococcus. It is part of the normal human microbiota, typically the skin microbiota, and less commonly the mucosal microbiota and also found in marine sponges. It is a facultative anaerobic bacteria. Although S. epidermidis is not usually pathogenic, patients with compromised immune systems are at risk of developing infection. These infections are generally hospital-acquired. S. epidermidis is a particular concern for people with catheters or other surgical implants because it is known to form biofilms that grow on these devices. Being part of the normal skin microbiota, S. epidermidis is a frequent contaminant of specimens sent to the diagnostic laboratory.
Virulence factors are cellular structures, molecules and regulatory systems that enable microbial pathogens to achieve the following:
Adenylate cyclase toxin is a virulence factor produced by some members of the genus Bordetella. Together with the pertussis toxin it is the most important virulence factor of the causative agent of whooping cough, Bordetella pertussis. Bordetella bronchiseptica and Bordetella parapertussis, also able to cause pertussis-like symptoms, also produce adenylate cyclase toxin. It is a toxin secreted by the bacteria to influence the host immune system.

Panton–Valentine leukocidin (PVL) is a cytotoxin—one of the β-pore-forming toxins. The presence of PVL is associated with increased virulence of certain strains (isolates) of Staphylococcus aureus. It is present in the majority of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) isolates studied and is the cause of necrotic lesions involving the skin or mucosa, including necrotic hemorrhagic pneumonia. PVL creates pores in the membranes of infected cells. PVL is produced from the genetic material of a bacteriophage that infects Staphylococcus aureus, making it more virulent.
Cytolysin refers to the substance secreted by microorganisms, plants or animals that is specifically toxic to individual cells, in many cases causing their dissolution through lysis. Cytolysins that have a specific action for certain cells are named accordingly. For instance, the cytolysins responsible for the destruction of red blood cells, thereby liberating hemoglobins, are named hemolysins, and so on. Cytolysins may be involved in immunity as well as in venoms.

Pore-forming proteins are usually produced by bacteria, and include a number of protein exotoxins but may also be produced by other organisms such as apple snails that produce perivitellin-2 or earthworms, who produce lysenin. They are frequently cytotoxic, as they create unregulated pores in the membrane of targeted cells.

Hemolysins or haemolysins are lipids and proteins that cause lysis of red blood cells by disrupting the cell membrane. Although the lytic activity of some microbe-derived hemolysins on red blood cells may be of great importance for nutrient acquisition, many hemolysins produced by pathogens do not cause significant destruction of red blood cells during infection. However, hemolysins are often capable of lysing red blood cells in vitro.
RNAIII is a stable 514 nt regulatory RNA transcribed by the P3 promoter of the Staphylococcus aureus quorum-sensing agr system ). It is the major effector of the agr regulon, which controls the expression of many S. aureus genes encoding exoproteins and cell wall associated proteins plus others encoding regulatory proteins The RNAIII transcript also encodes the 26 amino acid δ-haemolysin peptide (Hld). RNAIII contains many stem loops, most of which match the Shine-Dalgarno sequence involved in translation initiation of the regulated genes. Some of these interactions are inhibitory, others stimulatory; among the former is the regulatory protein Rot. In vitro, RNAIII is expressed post exponentially, inhibiting translation of the surface proteins, notably protein A, while stimulating that of the exoproteins, many of which are tissue-degrading enzymes or cytolysins. Among the latter is the important virulence factor, α-hemolysin (Hla), whose translation RNAIII activates by preventing the formation of an inhibitory foldback loop in the hla mRNA leader.

Alpha-toxin, also known as alpha-hemolysin (Hla), is the major cytotoxic agent released by bacterium Staphylococcus aureus and the first identified member of the pore forming beta-barrel toxin family. This toxin consists mostly of beta sheets (68%) with only about 10% alpha helices. The hly gene on the S. aureus chromosome encodes the 293 residue protein monomer, which forms heptameric units on the cellular membrane to form a complete beta barrel pore. This structure allows the toxin to perform its major function, development of pores in the cellular membrane, eventually causing cell death.
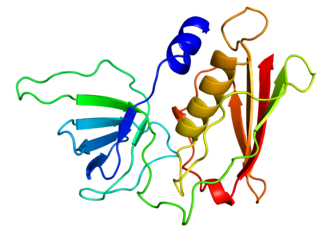
Toxic shock syndrome toxin-1 (TSST-1) is a superantigen with a size of 22 kDa produced by 5 to 25% of Staphylococcus aureus isolates. It causes toxic shock syndrome (TSS) by stimulating the release of large amounts of interleukin-1, interleukin-2 and tumor necrosis factor. In general, the toxin is not produced by bacteria growing in the blood; rather, it is produced at the local site of an infection, and then enters the blood stream.
Phenol-soluble modulins (PSMs) are a family of small proteins, that carry out a variety of functions, including acting as toxins, assisting in biofilm formation, and colony spreading. PSMs are produced by Staphylococcus bacteria including Methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus epidermidis. Many PSMs are encoded within the core genome and can play an important virulence factor. PSMs were first discovered in S. epidermidis by Seymour Klebanoff via hot-phenol extraction and were described as a pro-inflammatory complex of three peptides. Since their initial discovery, numerous roles of PSMs have been identified. However, due in part to the small size of many PSMs, they have largely gone unnoticed until recent years.
The RTX toxin superfamily is a group of cytolysins and cytotoxins produced by bacteria. There are over 1000 known members with a variety of functions. The RTX family is defined by two common features: characteristic repeats in the toxin protein sequences, and extracellular secretion by the type I secretion systems (T1SS). The name RTX refers to the glycine and aspartate-rich repeats located at the C-terminus of the toxin proteins, which facilitate export by a dedicated T1SS encoded within the rtx operon.

Aureolysin is an extracellular metalloprotease expressed by Staphylococcus aureus. This protease is a major contributor to the bacterium's virulence, or ability to cause disease, by cleaving host factors of the innate immune system as well as regulating S. aureus secreted toxins and cell wall proteins. To catalyze its enzymatic activities, aureolysin requires zinc and calcium which it obtains from the extracellular environment within the host.
Staphylococcus pseudintermedius is a gram-positive spherically shaped bacterium of the genus Staphylococcus found worldwide. It is primarily a pathogen for domestic animals, but has been known to affect humans as well. S. pseudintermedius is an opportunistic pathogen that secretes immune-modulating virulence factors, has many adhesion factors, and the potential to create biofilms, all of which help to determine the pathogenicity of the bacterium. Diagnoses of S. pseudintermedius have traditionally been made using cytology, plating, and biochemical tests. More recently, molecular technologies like MALDI-TOF, DNA hybridization and PCR have become preferred over biochemical tests for their more rapid and accurate identifications. This includes the identification and diagnosis of antibiotic resistant strains.
Andreas Peschel is a German microbiologist and an expert in bacterial pathogens. Peschel is Head of the Infection Biology Department at the University of Tübingen, Germany.
The SprA1/SPrA1as toxin/antitoxin system identified in Staphylococcus aureus, belongs to the Type I system encoding toxin protein: SprA1 and antitoxin RNA: SprA1as. The SprA1as postranscriptionally regulates SprA1 encoding small membrane damaging protein PepA1.
Accessory gene regulator (agr) is a complex 5 gene locus that is a global regulator of virulence in Staphylococcus aureus. It encodes a two-component transcriptional quorum-sensing (QS) system activated by an autoinducing, thiolactone-containing cyclic peptide (AIP).
References
- ↑ Nolte FS, Kapral FA (March 1981). "Immunogenicity of Staphylococcus aureus delta-toxin". Infection and Immunity. 31 (3): 1251–60. PMC 351449 . PMID 7014461.
- ↑ Murray PR, Rosenthal KS, Pfaller MA (2009) [1990]. Medical Microbiology (6th ed.). Philadelphia: Mosby. p. 213. ISBN 978-0-323-05470-6.
- ↑ Cheung GY, Yeh AJ, Kretschmer D, Duong AC, Tuffuor K, Fu CL, Joo HS, Diep BA, Li M, Nakamura Y, Nunez G, Peschel A, Otto M (December 2015). "Functional characteristics of the Staphylococcus aureus δ-toxin allelic variant G10S". Scientific Reports. 5 (1): 18023. doi:10.1038/srep18023. PMC 4674873 . PMID 26658455.
- ↑ Bloes DA, Haasbach E, Hartmayer C, Hertlein T, Klingel K, Kretschmer D, Planz O, Peschel A (December 2017). "Phenol-Soluble Modulin Peptides Contribute to Influenza A Virus-Associated Staphylococcus aureus Pneumonia". Infection and Immunity. 85 (12): e00620–17. doi:10.1128/IAI.00620-17. PMC 5695099 . PMID 28893917.
- 1 2 3 4 5 Otto M (February 2014). "Staphylococcus aureus toxins". Current Opinion in Microbiology. 17: 32–7. doi:10.1016/j.mib.2013.11.004. PMC 3942668 . PMID 24581690.
- ↑ Universal protein resource accession number P0C1V1 for "Delta-hemolysin" at UniProt.
- ↑ Dinges MM, Orwin PM, Schlievert PM (January 2000). "Exotoxins of Staphylococcus aureus". Clinical Microbiology Reviews. 13 (1): 16–34, table of contents. doi:10.1128/CMR.13.1.16. PMC 88931 . PMID 10627489.
- ↑ Schreiner J, Kretschmer D, Klenk J, Otto M, Bühring HJ, Stevanovic S, Wang JM, Beer-Hammer S, Peschel A, Autenrieth SE (April 2013). "Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells". Journal of Immunology. 190 (7): 3417–26. doi:10.4049/jimmunol.1202563. PMC 3608756 . PMID 23460735.
- ↑ Thelestam M, Möllby R, Wadström T (December 1973). "Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and HeLa cells: evaluation of a new quantitative as say for measuring cell damage". Infection and Immunity. 8 (6): 938–46. PMC 422954 . PMID 4784889.
- ↑ Kapral FA (January 1976). "Effect of fatty acids on Staphylococcus aureus delta-toxin hemolytic activity". Infection and Immunity. 13 (1): 114–9. PMC 420584 . PMID 1248865.