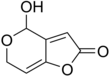
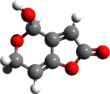
Aflatoxins are various poisonous carcinogens and mutagens that are produced by certain molds, particularly Aspergillus species. The fungi grow in soil, decaying vegetation and various staple foodstuffs and commodities such as hay, sweetcorn, wheat, millet, sorghum, cassava, rice, chili peppers, cottonseed, peanuts, tree nuts, sesame seeds, sunflower seeds, and various spices. In short, the relevant fungi grow on almost any crop or food. When such contaminated food is processed or consumed, the aflatoxins enter the general food supply. They have been found in both pet and human foods, as well as in feedstocks for agricultural animals. Animals fed contaminated food can pass aflatoxin transformation products into eggs, milk products, and meat. For example, contaminated poultry feed is the suspected source of aflatoxin-contaminated chicken meat and eggs in Pakistan.
A mycotoxin is a toxic secondary metabolite produced by fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops.

T-2 mycotoxin is a trichothecene mycotoxin. It is a naturally occurring mold byproduct of Fusarium spp. fungus which is toxic to humans and animals. The clinical condition it causes is alimentary toxic aleukia and a host of symptoms related to organs as diverse as the skin, airway, and stomach. Ingestion may come from consumption of moldy whole grains. T-2 can be absorbed through human skin. Although no significant systemic effects are expected after dermal contact in normal agricultural or residential environments, local skin effects can not be excluded. Hence, skin contact with T-2 should be limited.

Fumonisin B1 is the most prevalent member of a family of toxins, known as fumonisins, produced by multiple species of Fusarium molds, such as Fusarium verticillioides, which occur mainly in maize (corn), wheat and other cereals. Fumonisin B1 contamination of maize has been reported worldwide at mg/kg levels. Human exposure occurs at levels of micrograms to milligrams per day and is greatest in regions where maize products are the dietary staple.

Ochratoxins are a group of mycotoxins produced by some Aspergillus species and some Penicillium species, especially P. verrucosum. Ochratoxin A is the most prevalent and relevant fungal toxin of this group, while ochratoxins B and C are of lesser importance.

Ochratoxin A—a toxin produced by different Aspergillus and Penicillium species — is one of the most-abundant food-contaminating mycotoxins. It is also a frequent contaminant of water-damaged houses and of heating ducts. Human exposure can occur through consumption of contaminated food products, particularly contaminated grain and pork products, as well as coffee, wine grapes, and dried grapes. The toxin has been found in the tissues and organs of animals, including human blood and breast milk. Ochratoxin A, like most toxic substances, has large species- and sex-specific toxicological differences.

Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminates long-stored food and it causes different toxic effects, like nephrotoxic, hepatotoxic and cytotoxic effects. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.
Mycotoxicology is the branch of mycology that focuses on analyzing and studying the toxins produced by fungi, known as mycotoxins. In the food industry it is important to adopt measures that keep mycotoxin levels as low as practicable, especially those that are heat-stable. These chemical compounds are the result of secondary metabolism initiated in response to specific developmental or environmental signals. This includes biological stress from the environment, such as lower nutrients or competition for those available. Under this secondary path the fungus produces a wide array of compounds in order to gain some level of advantage, such as incrementing the efficiency of metabolic processes to gain more energy from less food, or attacking other microorganisms and being able to use their remains as a food source.
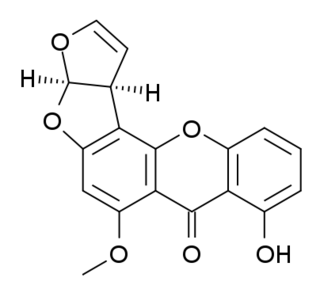
Sterigmatocystin is a polyketide mycotoxin produced by certain species of Aspergillus. The toxin is naturally found in some cheeses.

Penicillium expansum is a psychrophilic blue mold that is common throughout the world in soil. It causes Blue Mold of apples, one of the most prevalent and economically damaging post-harvest diseases of apples.
Mycoestrogens are xenoestrogens produced by fungi. They are sometimes referred to as mycotoxins. Among important mycoestrogens are zearalenone, zearalenol and zearalanol. Although all of these can be produced by various Fusarium species, zearalenol and zearalanol may also be produced endogenously in ruminants that have ingested zearalenone. Alpha-zearalanol is also produced semisynthetically, for veterinary use; such use is prohibited in the European Union.

3-MCPD (3-monochloropropane-1,2-diol or 3-chloropropane-1,2-diol) is an organic chemical compound with the formula HOCH2CH(OH)CH2Cl. It is a colorless liquid. It is a versatile multifunctional building block. The compound has attracted attention as the most common member of chemical food contaminants known as chloropropanols. It is suspected to be carcinogenic in humans.

Methiocarb is a carbamate pesticide which is used as an insecticide, bird repellent, acaricide and molluscicide since the 1960s. Methiocarb has contact and stomach action on mites and neurotoxic effects on molluscs. Seeds treated with methiocarb also affect birds. Other names for methiocarb are mesurol and mercaptodimethur.
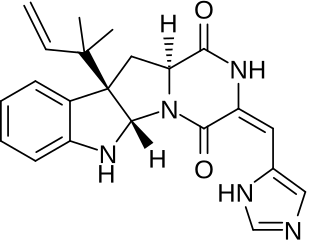
Roquefortine C is a mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by various fungi, particularly species from the genus Penicillium. It was first isolated from a strain of Penicillium roqueforti, a species commercially used as a source of proteolytic and lipolytic enzymes during maturation of the blue-veined cheeses, Roquefort, Danish Blue, Stilton and Gorgonzola.

Aflatoxin B1 is an aflatoxin produced by Aspergillus flavus and A. parasiticus. It is a very potent carcinogen with a TD50 3.2 μg/kg/day in rats. This carcinogenic potency varies across species with some, such as rats and monkeys, seemingly much more susceptible than others. Aflatoxin B1 is a common contaminant in a variety of foods including peanuts, cottonseed meal, corn, and other grains; as well as animal feeds. Aflatoxin B1 is considered the most toxic aflatoxin and it is highly implicated in hepatocellular carcinoma (HCC) in humans. In animals, aflatoxin B1 has also been shown to be mutagenic, teratogenic, and to cause immunosuppression. Several sampling and analytical methods including thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), mass spectrometry, and enzyme-linked immunosorbent assay (ELISA), among others, have been used to test for aflatoxin B1 contamination in foods. According to the Food and Agriculture Organization (FAO), a division of the United Nations, the worldwide maximum tolerated levels of aflatoxin B1 was reported to be in the range of 1–20 μg/kg (or .001 ppm - 1 part-per-billion) in food, and 5–50 μg/kg (.005 ppm) in dietary cattle feed in 2003.
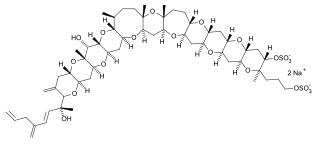
Yessotoxins are a group of lipophilic, sulfur bearing polyether toxins that are related to ciguatoxins. They are produced by a variety of dinoflagellates, most notably Lingulodinium polyedrum and Gonyaulax spinifera.
Tolerable weekly intake (TWI) estimates the amount per unit body weight of a potentially harmful substance or contaminant in food or water that can be ingested over a lifetime without risk of adverse health effects. TWI is generally preceded by "provisional" to indicate insufficient data exists, increasing uncertainty. The term TWI should be reserved for when there is a well-established and internationally accepted tolerance, backed by sound and uncontested data. Although similar in concept to tolerable daily intake (TDI), which is of the same derivation of acceptable daily intakes (ADIs), TWI accounts for contaminants that do not clear the body quickly and may accumulate within the body over a period of time. An example is heavy metals such as arsenic, cadmium, lead, and mercury. The concept of TWI takes into account daily variations in human consumption patterns.
Penicillium verrucosum is a psychrophilic fungus which was discovered in Belgium and introduced by Dierckx in 1901. Six varieties of this species have been recognized based primarily on differences in colony colour: P. verrucosum var. album, P. verrucosum var. corymbiferum, P. verrucosum var. cyclopium, P. verrucosum var. ochraceum, P. verrucosum var. melanochlorum and P. verrucosum var. verrucosum. This fungus has important implications in food, specifically for grains and other cereal crops on which it grows. Its growth is carefully regulated in order to reduce food spoilage by this fungi and its toxic products. The genome of P. verrucosum has been sequenced and the gene clusters for the biosyntheses of its mycotoxins have been identified.

Nivalenol (NIV) is a mycotoxin of the trichothecene group. In nature it is mainly found in fungi of the Fusarium species. The Fusarium species belongs to the most prevalent mycotoxin producing fungi in the temperate regions of the northern hemisphere, therefore making them a considerable risk for the food crop production industry.

Aflatoxin M1 is a chemical compound of the aflatoxin class, a group of mycotoxins produced by three species of Aspergillus – Aspergillus flavus, Aspergillus parasiticus, and the rare Aspergillus nomius – which contaminate plant and plant products.