
A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.
Calcicludine (CaC) is a protein toxin from the venom of the green mamba that inhibits high-voltage-activated calcium channels, especially L-type calcium channels.
Taicatoxin (TCX) is a snake toxin that blocks voltage-dependent L-type calcium channels and small conductance Ca2+-activated K+ channels. The name taicatoxin (TAIpan + CAlcium + TOXIN) is derived from its natural source, the taipan snake, the site of its action, calcium channels, and from its function as a toxin. Taicatoxin was isolated from the venom of Australian taipan snake, Oxyuranus scutellatus scutellatus. TCX is a secreted protein, produced in the venom gland of the snake.
Kurtoxin is a toxin found in the venom of the South African scorpion Parabuthus transvaalicus. It affects the gating of voltage-gated sodium channels and calcium channels.
Tityustoxin is a toxin found in the venom of scorpions from the subfamily Tityinae. By binding to voltage-dependent sodium ion channels and potassium channels, they cause sialorrhea, lacrimation and rhinorrhea.
Taipoxin is a potent myo- and neurotoxin that was isolated from the venom of the coastal taipan Oxyuranus scutellatus or also known as the common taipan. Taipoxin like many other pre-synaptic neurotoxins are phospholipase A2 (PLA2) toxins, which inhibit/complete block the release of the motor transmitter acetylcholine and lead to death by paralysis of the respiratory muscles (asphyxia). It is the most lethal neurotoxin isolated from any snake venom to date.

Agatoxins are a class of chemically diverse polyamine and peptide toxins which are isolated from the venom of various spiders. Their mechanism of action includes blockade of glutamate-gated ion channels, voltage-gated sodium channels, or voltage-dependent calcium channels. Agatoxin is named after the funnel web spider which produces a venom containing several agatoxins. There are different agatoxins. The ω-agatoxins are approximately 100 amino acids in length and are antagonists of voltage-sensitive calcium channels and also block the release of neurotransmitters. For instance, the ω-agatoxin 1A is a selective blocker and will block L-type calcium channels whereas the ω-agatoxin 4B will inhibit voltage sensitive P-type calcium channels. The μ-agatoxins only act on insect voltage-gated sodium channels.
Ablomin is a toxin present in the venom of the Japanese Mamushi snake, which blocks L-type voltage-gated calcium channels.
Piscivorin is a component of snake venom secreted by the Eastern Cottonmouth. It is a member of the cysteine-rich secretory protein (CRISP) family, which blocks voltage-dependent calcium channels.
Latisemin is a cysteine-rich secretory protein that can be isolated from the venom of the Black-banded sea krait, a sea snake indigenous to the warmer waters of the western Pacific Ocean. It is a toxin that inhibits cyclic nucleotide-gated ion channels and blocks L-type calcium channels, thereby reducing smooth muscle contraction.
Hanatoxin is a toxin found in the venom of the Grammostola spatulata tarantula. The toxin is mostly known for inhibiting the activation of voltage-gated potassium channels, most specifically Kv4.2 and Kv2.1, by raising its activation threshold.
Huwentoxins (HWTX) are a group of neurotoxic peptides found in the venom of the Chinese bird spider Haplopelma schmidti. The species was formerly known as Haplopelma huwenum, Ornithoctonus huwena and Selenocosmia huwena. While structural similarity can be found among several of these toxins, HWTX as a group possess high functional diversity.

Mambalgins are peptides found in the venom of the black mamba, an elapid snake. Mambalgins are members of the three-finger toxin (3FTx) protein family and have the characteristic three-finger protein fold. First reported by French researchers in 2012, mambalgins are unusual members of the 3FTx family in that they have the in vivo effect of causing analgesia without apparent toxicity. Their mechanism of action is potent inhibition of acid-sensing ion channels.

Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).
GTx1-15 is a toxin from the Chilean tarantula venom that acts as both a voltage-gated calcium channel blocker and a voltage-gated sodium channel blocker.

Noxiustoxin (NTX) is a toxin from the venom of the Mexican scorpion Centruroides noxius Hoffmann which block voltage-dependent potassium channels and calcium-activated potassium channels.
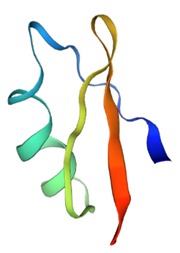
OdK2 is a toxin found in the venom of the Iranian scorpion Odonthobuthus doriae. It belongs to the α-KTx family, and selectively blocks the voltage-gated potassium channel Kv1.3 (KCNA3).
U7-ctenitoxin-Pn1a (or U7-CNTX-Pn1a for short) is a neurotoxin that blocks TRPV1 channels, and can exhibit analgestic effects. It is naturally found in the venom of Phoneutria nigriventer.
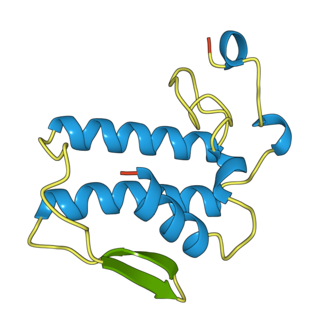
Notexin is a toxin produced by the tiger snake (Notechis scutatus). It is a myotoxic and presynaptic, neurotoxic phospholipase A2 (PLA2s). These are enzymes that hydrolyze the bond between a fatty acid tail and glycerol in fatty acids on the 2-position.
Phα1β is a peptide toxin that blocks various types of voltage-gated calcium channels (VGCCs) and is a specific receptor antagonist of the TRPA1 cation channel. The peptide is derived from the venom of the armed spider Phoneutria nigriventer and possesses wide-ranging analgesic and anti-nociceptive effects in animal models.







