A mycotoxin is a toxic secondary metabolite produced by fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.

Fusarium is a large genus of filamentous fungi, part of a group often referred to as hyphomycetes, widely distributed in soil and associated with plants. Most species are harmless saprobes, and are relatively abundant members of the soil microbial community. Some species produce mycotoxins in cereal crops that can affect human and animal health if they enter the food chain. The main toxins produced by these Fusarium species are fumonisins and trichothecenes. Despite most species apparently being harmless, some Fusarium species and subspecific groups are among the most important fungal pathogens of plants and animals.

T-2 mycotoxin is a trichothecene mycotoxin. It is a naturally occurring mold byproduct of Fusarium spp. fungus which is toxic to humans and other animals. The clinical condition it causes is alimentary toxic aleukia and a host of symptoms related to organs as diverse as the skin, airway, and stomach. Ingestion may come from consumption of moldy whole grains. T-2 can be absorbed through human skin. Although no significant systemic effects are expected after dermal contact in normal agricultural or residential environments, local skin effects can not be excluded. Hence, skin contact with T-2 should be limited.

Fumonisin B1 is the most prevalent member of a family of toxins, known as fumonisins, produced by multiple species of Fusarium molds, such as Fusarium verticillioides, which occur mainly in maize (corn), wheat and other cereals. Fumonisin B1 contamination of maize has been reported worldwide at mg/kg levels. Human exposure occurs at levels of micrograms to milligrams per day and is greatest in regions where maize products are the dietary staple.
Yellow rain was a 1981 political incident in which the United States Secretary of State Alexander Haig accused the Soviet Union of supplying T-2 mycotoxin to the communist states in Vietnam, Laos and Cambodia for use in counterinsurgency warfare. Refugees described many different forms of "attacks", including a sticky yellow liquid falling from planes or helicopters, which was dubbed "yellow rain". The U.S. government alleged that over ten thousand people had been killed in attacks using these supposed chemical weapons. The Soviets denied these claims and an initial United Nations investigation was inconclusive.

The trichothecenes are a large family of chemically related mycotoxins. They are produced by various species of Fusarium, Myrothecium,Trichoderma/Podostroma, Trichothecium, Cephalosporium, Verticimonosporium, and Stachybotrys. Chemically, trichothecenes are a class of sesquiterpenes.

Zearalenone (ZEN), also known as RAL and F-2 mycotoxin, is a potent estrogenic metabolite produced by some Fusarium and Gibberella species. Specifically, the Gibberella zeae, the fungal species where zearalenone was initially detected, in its asexual/anamorph stage is known as Fusarium graminearum. Several Fusarium species produce toxic substances of considerable concern to livestock and poultry producers, namely deoxynivalenol, T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS) and zearalenone. Particularly, ZEN is produced by Fusarium graminearum, Fusarium culmorum, Fusarium cerealis, Fusarium equiseti, Fusarium verticillioides, and Fusarium incarnatum. Zearalenone is the primary toxin that binds to estrogen receptors, causing infertility, abortion or other breeding problems, especially in swine. Often, ZEN is detected together with deoxynivalenol in contaminated samples and its toxicity needs to be considered in combination with the presence of other toxins.

Beauvericin is a depsipeptide with antibiotic and insecticidal effects belonging to the enniatin family. It was isolated from the fungus Beauveria bassiana, but is also produced by several other fungi, including several Fusarium species; it may therefore occur in grain contaminated with these fungi. Beauvericin is active against Gram-positive bacteria and mycobacteria, and is also capable of inducing programmed cell death in mammals.

Fumonisin B2 is a fumonisin mycotoxin produced by the fungi Fusarium verticillioides and Aspergillus niger.

The fumonisins are a group of mycotoxins derived from Fusarium and their Liseola section. They have strong structural similarity to sphinganine, the backbone precursor of sphingolipids.
Mycotoxicology is the branch of mycology that focuses on analyzing and studying the toxins produced by fungi, known as mycotoxins. In the food industry it is important to adopt measures that keep mycotoxin levels as low as practicable, especially those that are heat-stable. These chemical compounds are the result of secondary metabolism initiated in response to specific developmental or environmental signals. This includes biological stress from the environment, such as lower nutrients or competition for those available. Under this secondary path the fungus produces a wide array of compounds in order to gain some level of advantage, such as incrementing the efficiency of metabolic processes to gain more energy from less food, or attacking other microorganisms and being able to use their remains as a food source.
Fusarium tricinctum is a fungal and plant pathogen of various plant diseases worldwide, especially in temperate regions. It is found on many crops in the world including malt barley, and cereals.

Fusarium verticillioides is the most commonly reported fungal species infecting maize. Fusarium verticillioides is the accepted name of the species, which was also known as Fusarium moniliforme. The species has also been described as mating population A of the Fusarium fujikuroi species complex. F. verticllioides produces the mutagenic chemical compound fusarin C. F. verticillioides produces a group of disease-causing mycotoxins—fumonisins—on infected kernels.
Mycoestrogens are xenoestrogens produced by fungi. They are sometimes referred to as mycotoxins. Among important mycoestrogens are zearalenone, zearalenol and zearalanol. Although all of these can be produced by various Fusarium species, zearalenol and zearalanol may also be produced endogenously in ruminants that have ingested zearalenone. Alpha-zearalanol is also produced semisynthetically, for veterinary use; such use is prohibited in the European Union.

Zeranol, or zearanol, also known as α-zearalanol or simply zearalanol, is a synthetic nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in fungi in the Fusarium genus and is used mainly as an anabolic agent in veterinary medicine.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxins. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.
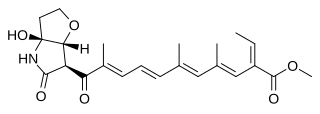
Fusarins are a class of mycotoxins produced mainly by fungi of the genus Fusarium, which can infect agriculturally important crops such as wheat, barley, oats, rye, and corn. Chemically, they are polyketides that are also derived from amino acids.
Glycerol dialkyl glycerol tetraether lipids (GDGTs) are a class of membrane lipids synthesized by archaea and some bacteria, making them useful biomarkers for these organisms in the geological record. Their presence, structure, and relative abundances in natural materials can be useful as proxies for temperature, terrestrial organic matter input, and soil pH for past periods in Earth history. Some structural forms of GDGT form the basis for the TEX86 paleothermometer. Isoprenoid GDGTs, now known to be synthesized by many archaeal classes, were first discovered in extremophilic archaea cultures. Branched GDGTs, likely synthesized by acidobacteriota, were first discovered in a natural Dutch peat sample in 2000.
Apiotrichum mycotoxinivorans is a yeast species purportedly useful in the detoxification of various mycotoxins. It was first isolated from the hindgut of the termite Mastotermes darwiniensis. It has been shown to detoxify mycotoxins such as ochratoxin A and zearalenone. It can occasionally become a human pathogen.










