
Unlike the action potential in skeletal muscle cells, the cardiac action potential is not initiated by nervous activity. Instead, it arises from a group of specialized cells known as pacemaker cells, that have automatic action potential generation capability. In healthy hearts, these cells form the cardiac pacemaker and are found in the sinoatrial node in the right atrium. They produce roughly 60–100 action potentials every minute. The action potential passes along the cell membrane causing the cell to contract, therefore the activity of the sinoatrial node results in a resting heart rate of roughly 60–100 beats per minute. All cardiac muscle cells are electrically linked to one another, by intercalated discs which allow the action potential to pass from one cell to the next. This means that all atrial cells can contract together, and then all ventricular cells.

In neuroscience, repolarization refers to the change in membrane potential that returns it to a negative value just after the depolarization phase of an action potential which has changed the membrane potential to a positive value. The repolarization phase usually returns the membrane potential back to the resting membrane potential. The efflux of potassium (K+) ions results in the falling phase of an action potential. The ions pass through the selectivity filter of the K+ channel pore.

hERG is a gene that codes for a protein known as Kv11.1, the alpha subunit of a potassium ion channel. This ion channel is best known for its contribution to the electrical activity of the heart: the hERG channel mediates the repolarizing IKr current in the cardiac action potential, which helps coordinate the heart's beating.
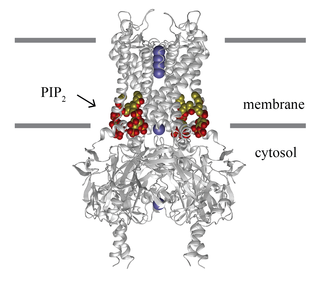
Inward-rectifier potassium channels (Kir, IRK) are a specific lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian and Alan Hodgkin in 1970 in skeletal muscle cells.

Potassium voltage-gated channel subfamily E member 1 is a protein that in humans is encoded by the KCNE1 gene.

Heteropodatoxins are peptide toxins from the venom of the giant crab spider Heteropoda venatoria, which block Kv4.2 voltage-gated potassium channels.

Potassium voltage-gated channel subfamily D member 2 is a protein that in humans is encoded by the KCND2 gene. It contributes to the cardiac transient outward potassium current (Ito1), the main contributing current to the repolarizing phase 1 of the cardiac action potential.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.

Potassium voltage-gated channel, Isk-related family, member 3 (KCNE3), also known as MinK-related peptide 2(MiRP2) is a protein that in humans is encoded by the KCNE3 gene.
Potassium voltage-gated channel subfamily D member 3 also known as Kv4.3 is a protein that in humans is encoded by the KCND3 gene. It contributes to the cardiac transient outward potassium current (Ito1), the main contributing current to the repolarizing phase 1 of the cardiac action potential.

Potassium voltage-gated channel subfamily E member 4, originally named MinK-related peptide 3 or MiRP3 when it was discovered, is a protein that in humans is encoded by the KCNE4 gene.

KCNE1-like also known as KCNE1L is a protein that in humans is encoded by the KCNE1L gene.
Phrixotoxins are peptide toxins derived from the venom of the Chilean copper tarantula Phrixotrichus auratus, also named Paraphysa scrofa. Phrixotoxin-1 and -2 block A-type voltage-gated potassium channels; phrixotoxin-3 blocks voltage-gated sodium channels. Similar toxins are found in other species, for instance the Chilean rose tarantula.

The cardiac transient outward potassium current (referred to as Ito1 or Ito ) is one of the ion currents across the cell membrane of heart muscle cells. It is the main contributing current during the repolarizing phase 1 of the cardiac action potential. It is a result of the movement of positively charged potassium (K+) ions from the intracellular to the extracellular space. Ito1 is complemented with Ito2 resulting from Cl− ions to form the transient outward current Ito.
Jingzhaotoxin proteins are part of a venom secreted by Chilobrachys jingzhao, the Chinese tarantula. and act as neurotoxins. There are several subtypes of jingzhaotoxin, which differ in terms of channel selectivity and modification characteristics. All subspecies act as gating modifiers of sodium channels and/or, to a lesser extent, potassium channels.

Heteroscodratoxin-1 is a neurotoxin produced by the venom glands of Heteroscodra maculata that shifts the activation threshold of voltage-gated potassium channels and the inactivation of Nav1.1 sodium channels to more positive potentials.

Guangxitoxin, also known as GxTX, is a peptide toxin found in the venom of the tarantula Plesiophrictus guangxiensis. It primarily inhibits outward voltage-gated Kv2.1 potassium channel currents, which are prominently expressed in pancreatic β-cells, thus increasing insulin secretion.
Hanatoxin is a toxin found in the venom of the Grammostola spatulata tarantula. The toxin is mostly known for inhibiting the activation of voltage-gated potassium channels, most specifically Kv4.2 and Kv2.1, by raising its activation threshold.

Stromatopelma is a genus of African tarantulas that was first described by Ferdinand Anton Franz Karsch in 1881. They are renowned for their potent venom that uses stromatoxin peptides to induce medically significant effects.
GiTx1 (β/κ-theraphotoxin-Gi1a) is a peptide toxin present in the venom of Grammostola iheringi. It reduces both inward and outward currents by blocking voltage-gated sodium and potassium channels, respectively.

















