Related Research Articles
Autolysins are endogenous lytic enzymes that break down the peptidoglycan components of biological cells which enables the separation of daughter cells following cell division. They are involved in cell growth, cell wall metabolism, cell division and separation, as well as peptidoglycan turnover and have similar functions to lysozymes.

Staphylococcus aureus is a gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe, meaning that it can grow without oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA). The bacterium is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.

Methicillin (USAN), also known as meticillin (INN), is a narrow-spectrum β-lactam antibiotic of the penicillin class.

An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. They can be chromosomally or plasmid encoded. They are heat labile, of low molecular weight and water-soluble. Enterotoxins are frequently cytotoxic and kill cells by altering the apical membrane permeability of the mucosal (epithelial) cells of the intestinal wall. They are mostly pore-forming toxins, secreted by bacteria, that assemble to form pores in cell membranes. This causes the cells to die.

Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of transpeptidase enzymes called DD-transpeptidases.

Sphingomyelin phosphodiesterase is a hydrolase enzyme that is involved in sphingolipid metabolism reactions. SMase is a member of the DNase I superfamily of enzymes and is responsible for breaking sphingomyelin (SM) down into phosphocholine and ceramide. The activation of SMase has been suggested as a major route for the production of ceramide in response to cellular stresses.
Cytolysin refers to the substance secreted by microorganisms, plants or animals that is specifically toxic to individual cells, in many cases causing their dissolution through lysis. Cytolysins that have a specific action for certain cells are named accordingly. For instance, the cytolysins responsible for the destruction of red blood cells, thereby liberating hemoglobins, are named hemolysins, and so on. Cytolysins may be involved in immunity as well as in venoms.

Pore-forming proteins are usually produced by bacteria, and include a number of protein exotoxins but may also be produced by other organisms such as apple snails that produce perivitellin-2 or earthworms, who produce lysenin. They are frequently cytotoxic, as they create unregulated pores in the membrane of targeted cells.

Hemolysins or haemolysins are lipids and proteins that cause lysis of red blood cells by disrupting the cell membrane. Although the lytic activity of some microbe-derived hemolysins on red blood cells may be of great importance for nutrient acquisition, many hemolysins produced by pathogens do not cause significant destruction of red blood cells during infection. However, hemolysins are often capable of lysing red blood cells in vitro.
RNAIII is a stable 514 nt regulatory RNA transcribed by the P3 promoter of the Staphylococcus aureus quorum-sensing agr system ). It is the major effector of the agr regulon, which controls the expression of many S. aureus genes encoding exoproteins and cell wall associated proteins plus others encoding regulatory proteins The RNAIII transcript also encodes the 26 amino acid δ-haemolysin peptide (Hld). RNAIII contains many stem loops, most of which match the Shine-Dalgarno sequence involved in translation initiation of the regulated genes. Some of these interactions are inhibitory, others stimulatory; among the former is the regulatory protein Rot. In vitro, RNAIII is expressed post exponentially, inhibiting translation of the surface proteins, notably protein A, while stimulating that of the exoproteins, many of which are tissue-degrading enzymes or cytolysins. Among the latter is the important virulence factor, α-hemolysin (Hla), whose translation RNAIII activates by preventing the formation of an inhibitory foldback loop in the hla mRNA leader.

Alpha-toxin, also known as alpha-hemolysin (Hla), is the major cytotoxic agent released by bacterium Staphylococcus aureus and the first identified member of the pore forming beta-barrel toxin family. This toxin consists mostly of beta sheets (68%) with only about 10% alpha helices. The hly gene on the S. aureus chromosome encodes the 293 residue protein monomer, which forms heptameric units on the cellular membrane to form a complete beta barrel pore. This structure allows the toxin to perform its major function, development of pores in the cellular membrane, eventually causing cell death.
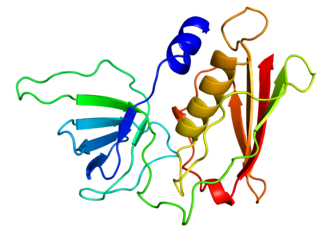
Toxic shock syndrome toxin-1 (TSST-1) is a superantigen with a size of 22 kDa produced by 5 to 25% of Staphylococcus aureus isolates. It causes toxic shock syndrome (TSS) by stimulating the release of large amounts of interleukin-1, interleukin-2 and tumor necrosis factor. In general, the toxin is not produced by bacteria growing in the blood; rather, it is produced at the local site of an infection, and then enters the blood stream.
Phenol-soluble modulins (PSMs) are a family of small proteins, that carry out a variety of functions, including acting as toxins, assisting in biofilm formation, and colony spreading. PSMs are produced by Staphylococcus bacteria including Methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus epidermidis. Many PSMs are encoded within the core genome and can play an important virulence factor. PSMs were first discovered in S. epidermidis by Seymour Klebanoff via hot-phenol extraction and were described as a pro-inflammatory complex of three peptides. Since their initial discovery, numerous roles of PSMs have been identified. However, due in part to the small size of many PSMs, they have largely gone unnoticed until recent years.
mecA is a gene found in bacterial cells which allows them to be resistant to antibiotics such as methicillin, penicillin and other penicillin-like antibiotics.
Staphylococcus aureus delta toxin is a toxin produced by Staphylococcus aureus. It has a wide spectrum of cytolytic activity.
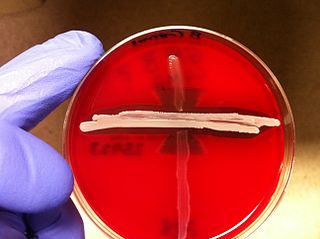
The CAMP test (Christie–Atkins–Munch-Petersen) is a test to identify group B β-hemolytic streptococci based on their formation of a substance, CAMP factor, that enlarges the area of hemolysis formed by the β-hemolysin elaborated from Staphylococcus aureus.
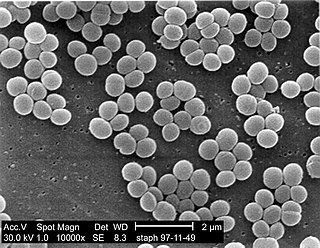
Staphylococcus is a genus of Gram-positive bacteria in the family Staphylococcaceae from the order Bacillales. Under the microscope, they appear spherical (cocci), and form in grape-like clusters. Staphylococcus species are facultative anaerobic organisms.
Clostridium perfringens beta toxin is one of the four major lethal protein toxins produced by Clostridium perfringens Type B and Type C strains. It is a necrotizing agent and it induces hypertension by release of catecholamine. It has been shown to cause necrotic enteritis in mammals and induces necrotizing intestinal lesions in the rabbit ileal loop model. C. perfringens beta toxin is susceptible to breakdown by proteolytic enzymes, particularly trypsin. Beta toxin is therefore highly lethal to infant mammals because of trypsin inhibitors present in the colostrum.

In the field of molecular biology, enterotoxin type B, also known as Staphylococcal enterotoxin B (SEB), is an enterotoxin produced by the gram-positive bacteria Staphylococcus aureus. It is a common cause of food poisoning, with severe diarrhea, nausea and intestinal cramping often starting within a few hours of ingestion. Being quite stable, the toxin may remain active even after the contaminating bacteria are killed. It can withstand boiling at 100 °C for a few minutes. Gastroenteritis occurs because SEB is a superantigen, causing the immune system to release a large amount of cytokines that lead to significant inflammation.
Staphylococcus schleiferi is a Gram-positive, cocci-shaped bacterium of the family Staphylococcaceae. It is facultatively anaerobic, coagulase-variable, and can be readily cultured on blood agar where the bacterium tends to form opaque, non-pigmented colonies and beta (β) hemolysis. There exists two subspecies under the species S. schleiferi: Staphylococcus schleiferi subsp. schleiferi and Staphylococcus schleiferi subsp. coagulans.
References
- ↑ Cifrian E, Guidry AJ, Bramley AJ, Norcross NL, Bastida-Corcuera FD, Marquardt WW (February 1996). "Effect of staphylococcal β toxin on the cytotoxicity, proliferation and adherence of Staphylococcus aureus to bovine mammary epithelial cells". Veterinary Microbiology. 48 (3–4): 187–98. doi: 10.1016/0378-1135(95)00159-X . PMID 9054116.
- ↑ Gaskin DK, Bohach GA, Schlievert PM, Hovde CJ (February 1997). "Purification of Staphylococcus aureus β-toxin: comparison of three isoelectric focusing methods". Protein Expression and Purification. 9 (1): 76–82. doi:10.1006/prep.1996.0664. PMID 9116505.
- ↑ Patrick R. Murray; Ken S. Rosenthal; Michael A. Pfaller (2009) [1990]. Medical Microbiology (6th ed.). Philadelphia: Mosby. p. 213. ISBN 978-0-323-05470-6.