
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. More rarely still, they may contain elements such as phosphorus, chlorine, and bromine.

Salamanders are a group of amphibians typically characterized by their lizard-like appearance, with slender bodies, blunt snouts, short limbs projecting at right angles to the body, and the presence of a tail in both larvae and adults. All ten extant salamander families are grouped together under the order Urodela from the group Caudata. Salamander diversity is highest in eastern North America, especially in the Appalachian Mountains; most species are found in the Holarctic realm, with some species present in the Neotropical realm.
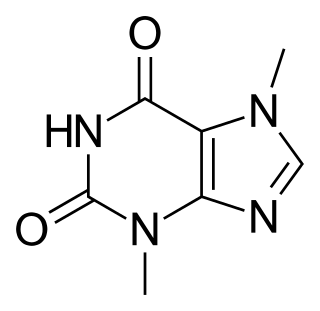
Theobromine, also known as xantheose, is the principal alkaloid of Theobroma cacao. Theobromine is slightly water-soluble (330 mg/L) with a bitter taste. In industry, theobromine is used as an additive and precursor to some cosmetics. It is found in chocolate, as well as in a number of other foods, including the leaves of the tea plant, and the kola nut. It is a white or colourless solid, but commercial samples can appear yellowish.

The Lissamphibia is a group of tetrapods that includes all modern amphibians. Lissamphibians consist of three living groups: the Salientia, the Caudata, and the Gymnophiona.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

The fire salamander is a common species of salamander found in Europe.
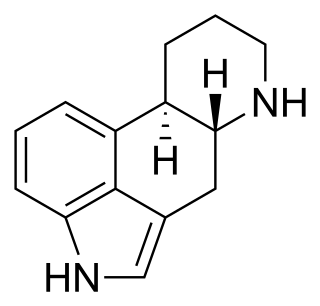
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.

The alpine salamander is a black salamander that can be found in the French Alps, and through the mountainous range in Europe. It is a member of the genus Salamandra. Their species name, atra, may be derived from the Latin ater, meaning dull black. The salamanders' coloration has evolved over time, as some species are completely monochrome black and others have yellow spotting and marks. Their life expectancy is at least 10 years. There are four subspecies of the alpine salamander, with varied distribution and physical coloration. Unlike other salamanders, whose larvae are developed in water, the alpine salamander and its subspecies are a fully terrestrial species in life and gestation. They give birth to live young.

Baeocystin is a zwitterionic alkaloid and analog of psilocybin. It is found as a minor compound in most psilocybin mushrooms together with psilocybin, norbaeocystin, aeruginascin, and psilocin. Baeocystin is an N-demethylated derivative of psilocybin, and a phosphorylated derivative of 4-HO-NMT (4-hydroxy-N-methyltryptamine). The structures at right illustrate baeocystin in its zwitterionic form.
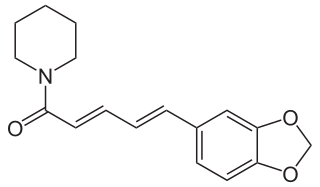
Piperine, possibly along with its isomer chavicine, is the compound responsible for the pungency of black pepper and long pepper. It has been used in some forms of traditional medicine.

A spermatophore or sperm ampulla is a capsule or mass containing spermatozoa created by males of various animal species, especially salamanders and arthropods, and transferred in entirety to the female's ovipore during reproduction. Spermatophores may additionally contain nourishment for the female, in which case it is called a nuptial gift, as in the instance of bush crickets. In the case of the toxic moth Utetheisa ornatrix, the spermatophore includes sperm, nutrients, and pyrrolizidine alkaloids which prevent predation because it is poisonous to most organisms. However, in some species such as the Edith's checkerspot butterfly, the "gift" provides little nutrient value. The weight of the spermatophore transferred at mating has little effect on female reproductive output.
Josephus Nicolaus Laurenti was an Austrian naturalist and zoologist of Italian origin.
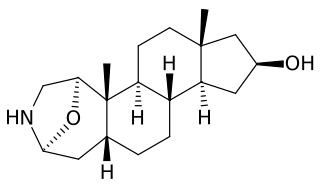
Samandarin or Samandarine is the main steroidal alkaloid secreted by the fire salamander (Salamandra salamandra). The compound is extremely toxic (LD50 = 70 µg/kg in mice). Poisoning can cause convulsions, respiratory paralysis, and eventual death. Samandarin is also believed to be the active ingredient in Salamander brandy, a Slovenian drink with purported hallucinogenic and aphrodisiac effects.
The Feist–Benary synthesis is an organic reaction between α-halo ketones and β-dicarbonyl compounds to produce substituted furan compounds. This condensation reaction is catalyzed by amines such as ammonia and pyridine. The first step in the ring synthesis is related to the Knoevenagel condensation. In the second step the enolate displaces an alkyl halogen in a nucleophilic aliphatic substitution.

The parotoid gland is an external skin gland on the back, neck, and shoulder of some frogs, and salamanders. It can secrete a number of milky alkaloid substances known collectively as bufotoxins, which act as neurotoxins to deter predation. These cutaneous glands are called parotoid as they are somewhat similarly positioned to mammalian parotid gland, although the latter have a different function, excreting saliva within the mouth rather than externally excreted defensive chemicals.

5-Bromo-DMT (5-bromo-N,N-dimethyltryptamine) is a psychedelic brominated indole alkaloid found in the sponges Smenospongia aurea and Smenospongia echina, as well as in Verongula rigida alongside 5,6-Dibromo-DMT and seven other alkaloids. It is the 5-bromo derivative of DMT, a psychedelic found in many plants and animals.
The salamander is an amphibian of the order Urodela which, as with many real creatures, often has been ascribed fantastic and sometimes occult qualities by pre-modern authors not possessed by the real organism. The legendary salamander is often depicted as a typical salamander in shape with a lizard-like form, but is usually ascribed an affinity with fire, sometimes specifically elemental fire.

Poisonous amphibians are amphibians that produce toxins to defend themselves from predators.

Steroidal alkaloids have the basic steroidal skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic cyclopentanoperhydrophenanthrene skeleton that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in Chonemorpha fragrans, 'chonemorphine' was used to treat intestinal infections in Wistar rats..

Isoquinoline alkaloids are natural products of the group of alkaloids, which are chemically derived from isoquinoline. They form the largest group among the alkaloids.

















