Fluoroacetate may refer to:
- Salts of fluoroacetic acid such as sodium fluoroacetate
- Fluoroacetate anion (FCH2CO−2), a conjugate base of fluoroacetic acid
- Esters of fluoroacetic acid
Fluoroacetate may refer to:

Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid.
SFA may refer to:

Sodium fluoroacetate, also known as compound 1080, is an organofluorine chemical compound with the chemical formula FCH2CO2Na. It is the sodium salt of fluoroacetic acid. It contains sodium cations Na+ and fluoroacetate anions FCH2CO−2. This colourless salt has a taste similar to that of table salt and is used as a rodenticide.

Aconitase is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process.

Fluoroacetic acid is a organofluorine compound with the chemical formula FCH2CO2H. It is a colorless solid that is noted for its relatively high toxicity. The conjugate base, fluoroacetate occurs naturally in at least 40 plants in Australia, Brazil, and Africa. It is one of only five known organofluorine-containing natural products.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

Bromofluoromethane is a mixed gaseous halomethane soluble in alcohol and very soluble in chloroform.
Bromoacetic acid is the chemical compound with the formula BrCH2CO2H. This colorless solid is a relatively strong alkylating agent. Bromoacetic acid and its esters are widely used building blocks in organic synthesis, for example, in pharmaceutical chemistry.
AF1 or AF-1 may refer to:
Fluoroacetamide is an organic compound based on acetamide with one fluorine atom replacing hydrogen on the methyl group. it is a metabolic poison which disrupts the citric acid cycle and was used as a rodenticide.
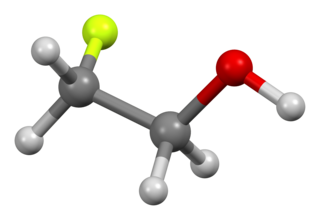
2-Fluoroethanol is the organic compound with the formula CH2FCH2OH. This colorless liquid is one of the simplest stable fluorinated alcohols. It was once used as a pesticide. The related difluoro- and trifluoroethanols are far less dangerous.

Fluoroacetyl chloride is an acyl chloride.
A dehalogenase is a type of enzyme that catalyzes the removal of a halogen atom from a substrate.

Fluorocitric acid is a fluorinated carboxylic acid derived from citric acid by substitution of one hydrogen by a fluorine atom. The appropriate anion is called fluorocitrate. Fluorocitrate is formed in two steps from fluoroacetate. Fluoroacetate is first converted to fluoroacetyl-CoA by acetyl-CoA synthetase in the mitochondria. Then fluoroacetyl-CoA condenses with oxaloacetate to form fluorocitrate. This step is catalyzed by citrate synthase. Flurocitrate is a metabolite of fluoroacetic acid and is very toxic because it is not processable using aconitase in the citrate cycle. The enzyme is inhibited and the cycle stops working.
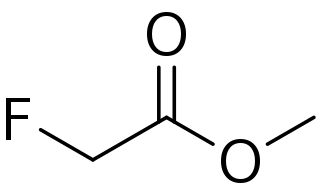
Methyl fluoroacetate (MFA) is an organic compound with the chemical formula FCH2CO2CH3. It is an extremely toxic methyl ester of fluoroacetic acid. It is a colorless, odorless liquid at room temperature. It is used as a laboratory chemical and as a rodenticide. Because of its extreme toxicity, MFA was studied for potential use as a chemical weapon.
The hydroxycarboxylic acid receptor (abbreviated HCA receptor and HCAR) family includes the following human proteins:

Fluoroethyl fluoroacetate, or more accurately 2-fluoroethyl fluoroacetate, is an organic compound with the chemical formula FCH2CO2CH2CH2F. It is the fluoroacetate ester of 2-fluoroethanol, or in other words, the 2-fluoroethyl ester of fluoroacetic acid. 2-Fluoroethyl fluoroacetate is two times more toxic than methyl fluoroacetate.
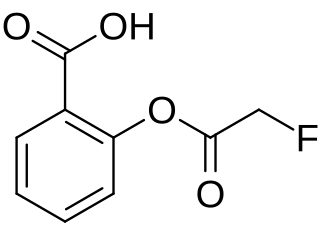
Fluoroaspirin is the fluoroacetate ester of salicylic acid. It is the fluoroacetate analog of aspirin. Like other fluoroacetate esters, fluoroaspirin is highly toxic.

2-Ethylhexyl fluoroacetate is an organic compound with the chemical formula FCH2CO2CH2CH(CH2CH3)CH2CH2CH2CH3. It is the fluoroacetate ester of 2-ethylhexanol, in other words, the 2-ethylhexyl ester of fluoroacetic acid. It can be produced by reaction of ethyl fluoroacetate with 2-ethylhexanol. 2-Ethylhexyl fluoroacetate is a liquid that is highly toxic by skin absorption.