
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.
Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Primary fibrinolysis is a normal body process, while secondary fibrinolysis is the breakdown of clots due to a medicine, a medical disorder, or some other cause.

Antithrombin (AT) is a small glycoprotein that inactivates several enzymes of the coagulation system. It is a 464-amino-acid protein produced by the liver. It contains three disulfide bonds and a total of four possible glycosylation sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manyfold by the anticoagulant drug heparin, which enhances the binding of antithrombin to factor IIa (thrombin) and factor Xa.

The partial thromboplastin time (PTT), also known as the activated partial thromboplastin time, is a blood test that characterizes coagulation of the blood. A historical name for this measure is the kaolin-cephalin clotting time (KCCT), reflecting kaolin and cephalin as materials historically used in the test. Apart from detecting abnormalities in blood clotting, partial thromboplastin time is also used to monitor the treatment effect of heparin, a widely prescribed drug that reduces blood's tendency to clot.
Lupus anticoagulant is an immunoglobulin that binds to phospholipids and proteins associated with the cell membrane. Its name is a partial misnomer, as it is actually a prothrombotic antibody in vivo. Lupus anticoagulant in living systems causes a decrease in clotting time. The name derives from their properties in vitro, as these antibodies increase coagulation times in laboratory tests such as the activated partial thromboplastin time (aPTT). Investigators speculate that the antibodies interfere with phospholipids used to induce in vitro coagulation. In vivo, the antibodies are thought to interact with platelet membrane phospholipids, increasing adhesion and aggregation of platelets, which accounts for the in vivo prothrombotic characteristics.

Protein S is a vitamin K-dependent plasma glycoprotein synthesized in the liver. In the circulation, Protein S exists in two forms: a free form and a complex form bound to complement protein C4b-binding protein (C4BP). In humans, protein S is encoded by the PROS1 gene. Protein S plays a role in coagulation.

Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.

Dilute Russell's viper venom time (dRVVT) is a laboratory test often used for detection of lupus anticoagulant (LA).
The prothrombinase complex consists of the serine protease, Factor Xa, and the protein cofactor, Factor Va. The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions. The prothrombinase complex catalyzes the conversion of prothrombin (Factor II), an inactive zymogen, to thrombin (Factor IIa), an active serine protease. The activation of thrombin is a critical reaction in the coagulation cascade, which functions to regulate hemostasis in the body. To produce thrombin, the prothrombinase complex cleaves two peptide bonds in prothrombin, one after Arg271 and the other after Arg320. Although it has been shown that Factor Xa can activate prothrombin when unassociated with the prothrombinase complex, the rate of thrombin formation is severely decreased under such circumstances. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 105-fold faster than can Factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.
Thromboelastography (TEG) is a method of testing the efficiency of blood coagulation. It is a test mainly used in surgery and anesthesiology, although increasingly used in resuscitations in Emergency Departments, intensive care units, and labor and delivery suites. More common tests of blood coagulation include prothrombin time (PT) and partial thromboplastin time (aPTT) which measure coagulation factor function, but TEG also can assess platelet function, clot strength, and fibrinolysis which these other tests cannot.

Activated protein C resistance (APCR) is a hypercoagulability characterized by a lack of a response to activated protein C (APC), which normally helps prevent blood from clotting excessively. This results in an increased risk of venous thrombosis, which resulting in medical conditions such as deep vein thrombosis and pulmonary embolism. The most common cause of hereditary APC resistance is factor V Leiden mutation.

Heparin cofactor II (HCII), a protein encoded by the SERPIND1 gene, is a coagulation factor that inhibits IIa, and is a cofactor for heparin and dermatan sulfate.
Purpura fulminans is an acute, often fatal, thrombotic disorder which manifests as blood spots, bruising and discolouration of the skin resulting from coagulation in small blood vessels within the skin and rapidly leads to skin necrosis and disseminated intravascular coagulation.

Ecarin clotting time (ECT) is a laboratory test used to monitor anticoagulation during treatment with hirudin, an anticoagulant medication which was originally isolated from leech saliva. Ecarin, the primary reagent in this assay, is derived from the venom of the saw-scaled viper, Echis carinatus.
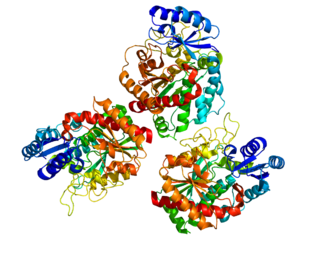
Carboxypeptidase B2 (CPB2), also known as carboxypeptidase U (CPU), plasma carboxypeptidase B (pCPB) or thrombin-activatable fibrinolysis inhibitor (TAFI), is an enzyme that, in humans, is encoded by the gene CPB2.
Thromboelastometry (TEM), previously named rotational thromboelastography (ROTEG) or rotational thromboelastometry (ROTEM), is an established viscoelastic method for hemostasis testing in whole blood. It is a modification of traditional thromboelastography (TEG).
Blood clotting tests are the tests used for diagnostics of the hemostasis system. Coagulometer is the medical laboratory analyzer used for testing of the hemostasis system. Modern coagulometers realize different methods of activation and observation of development of blood clots in blood or in blood plasma.
A thrombin generation assay (TGA) or thrombin generation test (TGT) is a global coagulation assay (GCA) and type of coagulation test which can be used to assess coagulation and thrombotic risk. It is based on the potential of a plasma to generate thrombin over time, following activation of coagulation via addition of phospholipids, tissue factor, and calcium. The results of the TGA can be output as a thrombogram or thrombin generation curve using computer software with calculation of thrombogram parameters.
The overall hemostatic potential (OHP) test is a global coagulation assay which can be used to measure coagulation. The OHP assay measures total fibrin generation in the presence of thrombin or tissue factor and tissue plasminogen activator (t-PA). It generates a fibrin time curve through the use of optical density measurement. This curve represents the balance between fibrin formation induced by thrombin or tissue factor and fibrinolysis induced by t-PA. The assay provides three parameters: overall coagulation potential (OCP), overall hemostatic potential (OHP), and overall fibrinolytic potential (OFP). OHP is the main parameter, while OCP and OFP are supplementary parameters to assess coagulation and fibrinolysis. One further parameter, clot lysis time (CLT), can also be determined. The OHP assay measures the integrated effect of procoagulant, anticoagulant, and fibrinolytic factors.











