
Platelets, also called thrombocytes, are a component of blood whose function is to react to bleeding from blood vessel injury by clumping, thereby initiating a blood clot. Platelets have no cell nucleus; they are fragments of cytoplasm that are derived from the megakaryocytes of the bone marrow or lung, which then enter the circulation. Circulating inactivated platelets are biconvex discoid (lens-shaped) structures, 2–3 µm in greatest diameter. Activated platelets have cell membrane projections covering their surface. Platelets are found only in mammals, whereas in other vertebrates, thrombocytes circulate as intact mononuclear cells.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.

Fibrinogen is a glycoprotein complex, made in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood clot. Fibrin clots function primarily to occlude blood vessels to stop bleeding. Fibrin also binds and reduces the activity of thrombin. This activity, sometimes referred to as antithrombin I, limits clotting. Fibrin also mediates blood platelet and endothelial cell spreading, tissue fibroblast proliferation, capillary tube formation, and angiogenesis and thereby promotes revascularization and wound healing.

Bradykinin is a peptide that promotes inflammation. It causes arterioles to dilate (enlarge) via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor and makes veins constrict, via prostaglandin F2, thereby leading to leakage into capillary beds, due to the increased pressure in the capillaries. Bradykinin is a physiologically and pharmacologically active peptide of the kinin group of proteins, consisting of nine amino acids.

Coagulation factor XII, also known as Hageman factor, is a plasma protein. It is the zymogen form of factor XIIa, an enzyme of the serine protease class. In humans, factor XII is encoded by the F12 gene.

Factor VIII (FVIII) is an essential blood-clotting protein, also known as anti-hemophilic factor (AHF). In humans, factor VIII is encoded by the F8 gene. Defects in this gene result in hemophilia A, a recessive X-linked coagulation disorder. Factor VIII is produced in liver sinusoidal cells and endothelial cells outside the liver throughout the body. This protein circulates in the bloodstream in an inactive form, bound to another molecule called von Willebrand factor, until an injury that damages blood vessels occurs. In response to injury, coagulation factor VIII is activated and separates from von Willebrand factor. The active protein interacts with another coagulation factor called factor IX. This interaction sets off a chain of additional chemical reactions that form a blood clot.
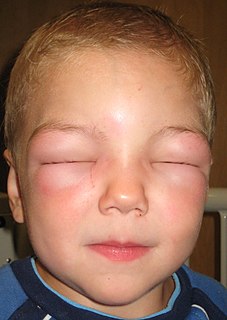
Angioedema is an area of swelling (edema) of the lower layer of skin and tissue just under the skin or mucous membranes. The swelling may occur in the face, tongue, larynx, abdomen, or arms and legs. Often it is associated with hives, which are swelling within the upper skin. Onset is typically over minutes to hours.
High-molecular-weight kininogen is a circulating plasma protein which participates in the initiation of blood coagulation, and in the generation of the vasodilator bradykinin via the kallikrein-kinin system. HMWK is inactive until it either adheres to binding proteins beneath an endothelium disrupted by injury, thereby initiating coagulation; or it binds to intact endothelial cells or platelets for functions other than coagulation.
The kinin–kallikrein system or simply kinin system is a poorly understood hormonal system with limited available research. It consists of blood proteins that play a role in inflammation, blood pressure control, coagulation and pain. Its important mediators bradykinin and kallidin are vasodilators and act on many cell types. Clinical symptoms include marked weakness, tachycardia, fever, leukocytosis and acceleration of ESR.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
Factor V is a protein of the coagulation system, rarely referred to as proaccelerin or labile factor. In contrast to most other coagulation factors, it is not enzymatically active but functions as a cofactor. Deficiency leads to predisposition for hemorrhage, while some mutations predispose for thrombosis.

Factor XI or plasma thromboplastin antecedent is the zymogen form of factor XIa, one of the enzymes of the coagulation cascade. Like many other coagulation factors, it is a serine protease. In humans, Factor XI is encoded by the F11 gene.
Tissue factor, also called platelet tissue factor, factor III, or CD142, is a protein encoded by the F3 gene, present in subendothelial tissue and leukocytes. Its role in the clotting process is the initiation of thrombin formation from the zymogen prothrombin. Thromboplastin defines the cascade that leads to the activation of factor X—the tissue factor pathway. In doing so, it has replaced the previously named extrinsic pathway in order to eliminate ambiguity.
Kallikreins are a subgroup of serine proteases, enzymes capable of cleaving peptide bonds in proteins. In humans, plasma kallikrein (KLKB1) has no known paralogue, while tissue kallikrein-related peptidases (KLKs) encode a family of fifteen closely related serine proteases. These genes are localised to chromosome 19q13, forming the largest contiguous cluster of proteases within the human genome. Kallikreins are responsible for the coordination of various physiological functions including blood pressure, semen liquefaction and skin desquamation.
Prekallikrein (PK), also known as Fletcher factor, is an 85,000 Mr serine protease that complexes with high-molecular-weight kininogen. PK is the precursor of plasma kallikrein, which is a serine protease that activates kinins. PK is cleaved to produce kallikrein by activated Factor XII.
Kininogens are precursor proteins for kinins, biologically active polypeptides involved in blood coagulation, vasodilation, smooth muscle contraction, inflammatory regulation, and the regulation of the cardiovascular and renal systems.

Plasma kallikrein is a protein that in humans is encoded by the KLKB1 gene.

Kininogen-1 (KNG1), also known as alpha-2-thiol proteinase inhibitor, Williams-Fitzgerald-Flaujeac factor or the HMWK-kallikrein factor is a protein that in humans is encoded by the KNG1 gene. Kininogen-1 is the precursor protein to high-molecular-weight kininogen (HMWK), low-molecular-weight kininogen (LMWK), and bradykinin.
Factor XII deficiency is a deficiency in the production of factor XII (FXII), a plasma glycoprotein and clotting factor that participates in the coagulation cascade and activates factor XI. FXII appears to be not essential for blood clotting, as individuals with this condition are usually asymptomatic and form blood clots in vivo. FXII deficiency tends to be identified during presurgical laboratory screening for bleeding disorders.














