
A spermatozoon is a motile sperm cell, or moving form of the haploid cell that is the male gamete. A spermatozoon joins an ovum to form a zygote.

For fertilization to happen between a sperm and egg cell, a sperm must first fuse with the plasma membrane and then penetrate the female egg cell to fertilize it. While the fusion of the sperm cell with the egg cell's plasma membrane is relatively straightforward, penetrating the egg's protective layers, such as the zona pellucida, presents a significant challenge. Therefore, sperm cells go through a process known as the acrosome reaction, which is the reaction that occurs in the acrosome of the sperm as it approaches the egg.

The zona pellucida is the specialized area surrounding mammalian oocytes (eggs). It is also known as an egg coat. The zona pellucida is essential for oocyte growth and fertilization.
Capacitation is the penultimate step in the maturation of mammalian spermatozoa and is required to render them competent to fertilize an oocyte. This step is a biochemical event; the sperm move normally and look mature prior to capacitation. In vivo, capacitation occurs after ejaculation, when the spermatozoa leave the vagina and enter the upper female reproductive tract. The uterus aids in the steps of capacitation by secreting sterol-binding albumin, lipoproteins, and proteolytic and glycosidasic enzymes such as heparin.
Hyperactivation is a type of sperm motility. Hyperactivated sperm motility is characterised by a high amplitude, asymmetrical beating pattern of the sperm tail (flagellum). This type of motility may aid in sperm penetration of the zona pellucida, which encloses the ovum.
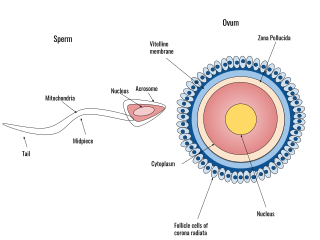
Human fertilization is the union of an egg and sperm, occurring primarily in the ampulla of the fallopian tube. The result of this union leads to the production of a fertilized egg called a zygote, initiating embryonic development. Scientists discovered the dynamics of human fertilization in the 19th century.

The cortical reaction is a process initiated during fertilization that prevents polyspermy, the fusion of multiple sperm with one egg. In contrast to the fast block of polyspermy which immediately but temporarily blocks additional sperm from fertilizing the egg, the cortical reaction gradually establishes a permanent barrier to sperm entry and functions as the main part of the slow block of polyspermy in many animals.
Decapacitation factor (DF) is composed of sperm surface-associated proteins which modulate the fertilizing ability of spermatozoa. Decapacitation is a reversible process that converts fertile, capacitated sperm to less-fertile uncapacitated sperm. This activity is achieved by interaction between cholesterol, phospholipids and fibronectin-like substances and delivered via small vesicles in seminal plasma. DF prevents onset of capacitation. Many DFs are released in secretions from the epididymis and accessory organs of the male reproductive system. However, some DFs have been identified that are located on the acrosome of sperm. Normally, capacitation is initiated through the loss of DF before the spermatozoa can perform the acrosomal reaction. Physiologically decapacitation will inhibit the acrosomal reaction as DFs reassociate onto the sperm surface. For example, one way this can be achieved is through spermatozoal membrane stabilization by maintaining physiological cholesterol/phospholipid ratio.

Protein C inhibitor is a serine protease inhibitor (serpin) that limits the activity of protein C.
Zona pellucida sperm-binding protein 3, also known as zona pellucida glycoprotein 3 (Zp-3) or the sperm receptor, is a ZP module-containing protein that in humans is encoded by the ZP3 gene. ZP3 is the glycoprotein in the zona pellucida most important for inducting the acrosome reaction of sperm cells at the beginning of fertilization.
Immunocontraception is the use of an animal's immune system to prevent it from fertilizing offspring. Contraceptives of this type are not currently approved for human use.

Glycodelin(GD) also known as human placental protein-14 (PP-14)progestogen-associated endometrial protein (PAEP) or pregnancy-associated endometrial alpha-2 globulin is a glycoprotein that inhibits cell immune function and plays an essential role in the pregnancy process. In humans is encoded by the PAEP gene.

Zona pellucida sperm-binding protein 2 is a protein that in humans is encoded by the ZP2 gene.

Zona pellucida sperm-binding protein 4, ZP-4 or avilesine, named after its discoverer Manuel Avilés Sánchez is a protein that in humans is encoded by the ZP4 gene.

Serine protease inhibitor Kazal-type 2 also known as acrosin-trypsin inhibitor is a protein that in humans is encoded by the SPINK2 gene.

Cysteine-rich secretory proteins, often abbreviated as CRISPs, are a group of glycoproteins. They are a subgroup of the CRISP, antigen 5 and Pr-1 (CAP) protein superfamily and also contain a domain related to the ShK toxins. They are substantially implicated in the functioning of the mammalian reproductive system. CRISPs are also found in a variety of snake venoms where they inhibit both smooth muscle contraction and cyclic nucleotide-gated ion channels.

The Kazal domain is an evolutionary conserved protein domain usually indicative of serine protease inhibitors. However, kazal-like domains are also seen in the extracellular part of agrins, which are not known to be protease inhibitors.

Cortical granules are regulatory secretory organelles found within oocytes and are most associated with polyspermy prevention after the event of fertilization. Cortical granules are found among all mammals, many vertebrates, and some invertebrates. Within the oocyte, cortical granules are located along the cortex, the region furthest from the cell's center. Following fertilization, a signaling pathway induces the cortical granules to fuse with the oocyte's cell membrane and release their contents into the oocyte's extracellular matrix. This exocytosis of cortical granules is known as the cortical reaction. In mammals, the oocyte's extracellular matrix includes a surrounding layer of perivitelline space, zona pellucida, and finally cumulus cells. Experimental evidence has demonstrated that the released contents of the cortical granules modify the oocyte's extracellular matrix, particularly the zona pellucida. This alteration of the zona pellucida components is known as the zona reaction. The cortical reaction does not occur in all mammals, suggesting the likelihood of other functional purposes for cortical granules. In addition to modifying the oocyte's extracellular matrix and establishing a block to polyspermy, the exocytosis of cortical granules may also contribute towards protection and support of the developing embryo during preimplantation. Once the cortical granules complete their functions, the oocyte does not replenish them.

Acrosin binding protein is a protein that in humans is encoded by the ACRBP gene.
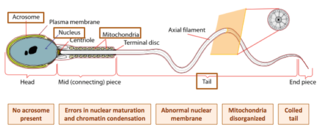
Globozoospermia is a rare and severe form of monomorphic teratozoospermia. This means that the spermatozoa show the same abnormality, and over 85% of spermatozoa in sperm have this abnormality. Globozoospermia is responsible for less than 0.1% of male infertility. It is characterised by round-headed spermatozoa without acrosomes, an abnormal nuclear membrane and midpiece defects. Affected males therefore suffer from either reduced fertility or infertility. Studies suggest that globozoospermia can be either total or partial, however it is unclear whether these two forms are variations on the same syndrome, or actually different syndromes.


















