
Gamma-glutamyltransferase is a transferase that catalyzes the transfer of gamma-glutamyl functional groups from molecules such as glutathione to an acceptor that may be an amino acid, a peptide or water. GGT plays a key role in the gamma-glutamyl cycle, a pathway for the synthesis and degradation of glutathione as well as drug and xenobiotic detoxification. Other lines of evidence indicate that GGT can also exert a pro-oxidant role, with regulatory effects at various levels in cellular signal transduction and cellular pathophysiology. This transferase is found in many tissues, the most notable one being the liver, and has significance in medicine as a diagnostic marker.

Leukotrienes are a family of eicosanoid inflammatory mediators produced in leukocytes by the oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxygenase.

Epoxide hydrolases (EHs), also known as epoxide hydratases, are enzymes that metabolize compounds that contain an epoxide residue; they convert this residue to two hydroxyl residues through an epoxide hydrolysis reaction to form diol products. Several enzymes possess EH activity. Microsomal epoxide hydrolase, soluble epoxide hydrolase, and the more recently discovered but not as yet well defined functionally, epoxide hydrolase 3 (EH3) and epoxide hydrolase 4 (EH4) are structurally closely related isozymes. Other enzymes with epoxide hydrolase activity include leukotriene A4 hydrolase, Cholesterol-5,6-oxide hydrolase, MEST (gene) (Peg1/MEST), and Hepoxilin-epoxide hydrolase. The hydrolases are distinguished from each other by their substrate preferences and, directly related to this, their functions.

Monoacylglycerol lipase is an enzyme that, in humans, is encoded by the MGLL gene. MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.

Leukotriene C4 (LTC4) is a leukotriene. LTC4 has been extensively studied in the context of allergy and asthma. In cells of myeloid origin such as mast cells, its biosynthesis is orchestrated by translocation to the nuclear envelope along with co-localization of cytosolic phospholipase A2 (cPLA2), arachidonate 5-lipoxygenase (5-LO), 5-lipoxygenase-activating protein (FLAP) and LTC4 synthase (LTC4S), which couples glutathione to an LTA4 intermediate. The MRP1 transporter then secretes cytosolic LTC4 and cell surface proteases further metabolize it by sequential cleavage of the γ-glutamyl and glycine residues off its glutathione segment, generating the more stable products LTD4 and LTE4. All three leukotrienes then bind at different affinities to two G-protein coupled receptors: CYSLTR1 and CYSLTR2, triggering pulmonary vasoconstriction and bronchoconstriction.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.

Leukotriene C4 synthase is an enzyme that in humans is encoded by the LTC4S gene.

Leukotriene A4 hydrolase, also known as LTA4H is a human gene. The protein encoded by this gene is a bifunctional enzyme which converts leukotriene A4 to leukotriene B4 and acts as an aminopeptidase.

In enzymology, a microsomal epoxide hydrolase (mEH) is an enzyme that catalyzes the hydrolysis reaction between an epoxide and water to form a diol.

Cysteinyl leukotriene receptor 1, also termed CYSLTR1, is a receptor for cysteinyl leukotrienes (LT). CYSLTR1, by binding these cysteinyl LTs contributes to mediating various allergic and hypersensitivity reactions in humans as well as models of the reactions in other animals.

Microsomal glutathione S-transferase 2 is an enzyme that in humans is encoded by the MGST2 gene.

Gamma-glutamyltransferase 1 (GGT1), also known as CD224, is a human gene.

Gamma-glutamyltransferase 5 is an enzyme that in humans is encoded by the GGT5 gene.

Dipeptidase 2 (DPEP2) is a protein which in humans is encoded by the DPEP2 gene.

Dipeptidase 3 (DPEP3) is a protein that in humans is encoded by the DPEP3 gene.

Ghk.
Gamma-D-glutamyl-meso-diaminopimelate peptidase is an enzyme. This enzyme catalyses the following chemical reaction
Glutathione hydrolase (EC 3.4.19.13, glutathionase, GGT, gamma-glutamyltranspeptidase) is an enzyme. This enzyme catalyses the following chemical reaction
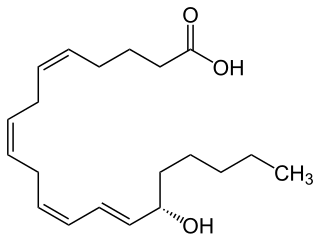
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
Eoxins are proposed to be a family of proinflammatory eicosanoids. They are produced by human eosinophils, mast cells, the L1236 Reed–Sternberg cell line derived from Hodgkin's lymphoma, and certain other tissues. These cells produce the eoxins by initially metabolizing arachidonic acid, an omega-6 (ω-6) fatty acid, via any enzyme possessing 15-lipoxygenase activity. The product of this initial metabolic step, 15(S)-hydroperoxyeicosatetraenoic acid, is then converted to a series of eoxins by the same enzymes that metabolize the 5-lipoxygenase product of arachidonic acid metabolism, i.e. 5-Hydroperoxy-eicosatetraenoic acid to a series of leukotrienes. That is, the eoxins are 14,15-disubstituted analogs of the 5,6-disubstituted leukotrienes.













