 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a686013 |
| Routes of administration | Intravenous |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.072.592 |
| Chemical and physical data | |
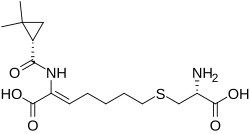
| Formula | C16H26N2O5S |
| Molar mass | 358.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cilastatin inhibits the human enzyme dehydropeptidase. [1]
Cilastatin is a therapeutic alternative on the World Health Organization's List of Essential Medicines. [2]