Aromatic compounds are those chemical compounds that contain one or more rings with pi electrons delocalized all the way around them. In contrast to compounds that exhibit aromaticity, aliphatic compounds lack this delocalization. The term "aromatic" was assigned before the physical mechanism determining aromaticity was discovered, and referred simply to the fact that many such compounds have a sweet or pleasant odour; however, not all aromatic compounds have a sweet odour, and not all compounds with a sweet odour are aromatic. Aromatic hydrocarbons, or arenes, are aromatic organic compounds containing solely carbon and hydrogen atoms. The configuration of six carbon atoms in aromatic compounds is called a "benzene ring", after the simple aromatic compound benzene, or a phenyl group when part of a larger compound.

In chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with pi bonds in resonance that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.
In chemistry, an open-chain compound or acyclic compound is a compound with a linear structure, rather than a cyclic one. An open-chain compound having no side chains is called a straight-chain compound. Many of the simple molecules of organic chemistry, such as the alkanes and alkenes, have both linear and ring isomers, that is, both acyclic and cyclic, with the latter often classified as aromatic. For those with 4 or more carbons, the linear forms can have straight-chain or branched-chain isomers. The lowercase prefix n- denotes the straight-chain isomer; for example, n-butane is straight-chain butane, whereas i-butane is isobutane. Cycloalkanes are isomers of alkenes, not of alkanes, because the ring's closure involves a C-C bond. Having no rings, all open-chain compounds are aliphatic.
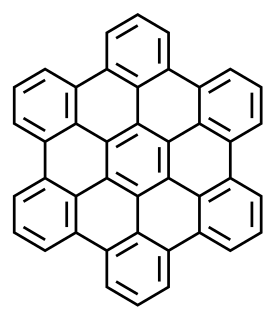
A polycyclic aromatic hydrocarbon (PAH) is a hydrocarbon—a chemical compound containing only carbon and hydrogen—that is composed of multiple aromatic rings. The group is a major subset of the aromatic hydrocarbons. The simplest of such chemicals are naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept.

Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

Azo compounds are compounds bearing the functional group diazenyl R−N=N−R′, in which R and R′ can be either aryl or alkyl.

Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.
Dihydroxybenzenes are organic chemical compounds in which two hydroxyl groups are substituted onto a benzene ring. These aromatic compounds are classed as phenols. There are three isomer: 1,2-dihydroxybenzene is commonly known as catechol, 1,3-dihydroxybenzene is commonly known as resorcinol, and 1,4-dihydroxybenzene is commonly known as hydroquinone.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.

1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.

Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry. The all-cis isomer (1), a fully convex decagon, would have bond angles of 144°, which creates large amounts of angle strain relative to the ideal 120° for sp2 atomic hybridization. Instead, the all-cis isomer can adopt a planar boat-like conformation (2) to relieve the angle strain. This is still unstable because of the relative higher strain in boat shaped compared to the next planar trans, cis, trans, cis, cis isomer (3). Yet even this isomer is also unstable, suffering from steric repulsion between the two internal hydrogen atoms. The nonplanar trans, cis, cis, cis, cis isomer (4) is the most stable of all the possible isomers.

Benzoxazole is an aromatic organic compound with a molecular formula C7H5NO, a benzene-fused oxazole ring structure, and an odor similar to pyridine. Although benzoxazole itself is of little practical value, many derivatives of benzoxazoles are commercially important.
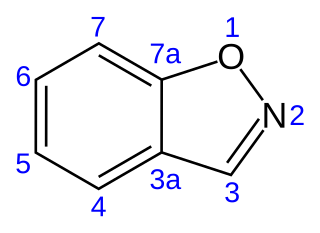
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure. The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.
p-Cymene is a naturally occurring aromatic organic compound. It is classified as an alkylbenzene related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group. p-Cymene is insoluble in water, but miscible with organic solvents.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic, in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an organic compound with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.

Peroxynitrous acid (HNO3) is a reactive nitrogen species (RNS). It is the conjugate acid of peroxynitrite (ONOO−). It has a pKa of approximately 6.8. It is formed in vivo from the diffusion-controlled reaction of nitrogen monoxide (ON•) and superoxide (O•−
2). It is an isomer of nitric acid and isomerises with a rate constant of k = 1.2 s−1, a process whereby up to 5% of hydroxyl and nitrogen dioxide radicals may be formed. It oxidises and nitrates aromatic compounds in low yield. The mechanism may involve a complex between the aromatic compound and ONOOH, and a transition from the cis- to the trans-configuration of ONOOH. Peroxynitrous acid is also important in atmospheric chemistry.
The molecular formula C7H5NO (molar mass: 119.12 g/mol, exact mass: 119.0371 u) may refer to:

 [1]
[1] 











