
Glutamate dehydrogenase is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typically, the α-ketoglutarate to glutamate reaction does not occur in mammals, as glutamate dehydrogenase equilibrium favours the production of ammonia and α-ketoglutarate. Glutamate dehydrogenase also has a very low affinity for ammonia, and therefore toxic levels of ammonia would have to be present in the body for the reverse reaction to proceed. However, in brain, the NAD+/NADH ratio in brain mitochondria encourages oxidative deamination. In bacteria, the ammonia is assimilated to amino acids via glutamate and aminotransferases. In plants, the enzyme can work in either direction depending on environment and stress. Transgenic plants expressing microbial GLDHs are improved in tolerance to herbicide, water deficit, and pathogen infections. They are more nutritionally valuable.
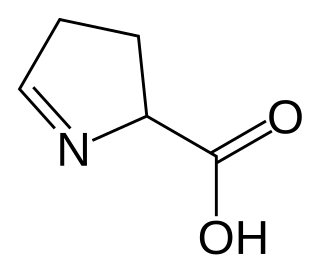
1-Pyrroline-5-carboxylic acid is a cyclic imino acid. Its conjugate base and anion is 1-pyrroline-5-carboxylate (P5C). In solution, P5C is in spontaneous equilibrium with glutamate-5-semialdhyde (GSA).

Hyperprolinemia is a condition which occurs when the amino acid proline is not broken down properly by the enzymes proline oxidase or pyrroline-5-carboxylate dehydrogenase, causing a buildup of proline in the body.

In enzymology, a succinate-semialdehyde dehydrogenase (SSADH) (EC 1.2.1.24) is an enzyme that catalyzes the chemical reaction
In enzymology, a succinylglutamate-semialdehyde dehydrogenase (EC 1.2.1.71) is an enzyme that catalyzes the chemical reaction

In enzymology, a (R,R)-butanediol dehydrogenase (EC 1.1.1.4) is an enzyme that catalyzes the chemical reaction
In enzymology, histidinol dehydrogenase (HIS4) (HDH) (EC 1.1.1.23) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-threonine 3-dehydrogenase (EC 1.1.1.103) is an enzyme that catalyzes the chemical reaction
In enzymology, a testosterone 17beta-dehydrogenase is an enzyme that catalyzes the chemical reaction between testosterone and androst-4-ene-3,17-dione. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor.

In enzymology, a 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35) is an enzyme that catalyzes the chemical reaction
In enzymology, 3-hydroxybutyrate dehydrogenase (EC 1.1.1.30) is an enzyme that catalyzes the chemical reaction:
In enzymology, a 1,6-dihydroxycyclohexa-2,4-diene-1-carboxylate dehydrogenase (EC 1.3.1.25) is an enzyme that catalyzes the chemical reaction
In enzymology, an aminobutyraldehyde dehydrogenase (EC 1.2.1.19) is an enzyme that catalyzes the chemical reaction

In enzymology, a betaine-aldehyde dehydrogenase (EC 1.2.1.8) is an enzyme that catalyzes the chemical reaction

In enzymology, proline dehydrogenase (PRODH) (EC 1.5.5.2, formerly EC 1.5.99.8) is an enzyme of the oxidoreductase family, active in the oxidation of L-proline to (S)-1-pyrroline-5-carboxylate during proline catabolism. The end product of this reaction is then further oxidized in a (S)-1-pyrroline-5-carboxylate dehydrogenase (P5CDH)-dependent reaction of the proline metabolism, or spent to produce ornithine, a crucial metabolite of ornithine and arginine metabolism. The systematic name of this enzyme class is L-proline:quinone oxidoreductase. Other names in common use include L-proline dehydrogenase, L-proline oxidase,and L-proline:(acceptor) oxidoreductase. It employs one cofactor, FAD, which requires riboflavin (vitamin B2).
In enzymology, a pyrroline-2-carboxylate reductase (EC 1.5.1.1) is an enzyme that catalyzes the chemical reaction

In enzymology, a pyrroline-5-carboxylate reductase (EC 1.5.1.2) is an enzyme that catalyzes the chemical reaction

Pyrroline-5-carboxylate reductase 1, mitochondrial is an enzyme that in humans is encoded by the PYCR1 gene.

Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ALDH4A1 gene.
Arginine and proline metabolism is one of the central pathways for the biosynthesis of the amino acids arginine and proline from glutamate. The pathways linking arginine, glutamate, and proline are bidirectional. Thus, the net utilization or production of these amino acids is highly dependent on cell type and developmental stage. Altered proline metabolism has been linked to metastasis formation in breast cancer.













