Related Research Articles

Collagen is the main structural protein in the extracellular matrix of a body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals. 25% to 35% of a mammalian body's protein content is collagen. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. The collagen helix is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis, while Vitamin E improves its production.

Ehlers–Danlos syndromes (EDS) are a group of 13 genetic connective-tissue disorders. Symptoms often include loose joints, joint pain, stretchy velvety skin, and abnormal scar formation. These may be noticed at birth or in early childhood. Complications may include aortic dissection, joint dislocations, scoliosis, chronic pain, or early osteoarthritis. The current classification was last updated in 2017, when a number of rarer forms of EDS were added.
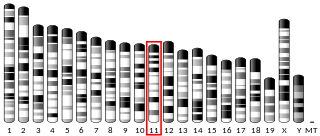
A disintegrin and metalloproteinase with thrombospondin motifs 2 (ADAM-TS2) also known as procollagen I N-proteinase is an enzyme that in humans is encoded by the ADAMTS2 gene.

Collagen, type I, alpha 1, also known as alpha-1 type I collagen, is a protein that in humans is encoded by the COL1A1 gene. COL1A1 encodes the major component of type I collagen, the fibrillar collagen found in most connective tissues, including cartilage.

Collagen alpha-2(XI) chain is a protein that in humans is encoded by the COL11A2 gene.
Fibrillogenesis is the development of fine fibrils normally present in collagen fibers of connective tissue. It is derived from the New Latin fibrilla and Greek genesis.

Sack–Barabas syndrome (SBS) is an older name for vascular Ehlers–Danlos syndrome (vEDS). It is a medical condition, a subset of Ehlers–Danlos syndrome which especially affects the body's vascular system, including blood vessels and organs, and makes them prone to rupture.
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the COL3A1 gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain.
Collagen IV is a type of collagen found primarily in the basal lamina. The collagen IV C4 domain at the C-terminus is not removed in post-translational processing, and the fibers link head-to-head, rather than in parallel. Also, collagen IV lacks the regular glycine in every third residue necessary for the tight, collagen helix. This makes the overall arrangement more sloppy with kinks. These two features cause the collagen to form in a sheet, the form of the basal lamina. Collagen IV is the more common usage, as opposed to the older terminology of "type-IV collagen". Collagen IV exists in all metazoan phyla, to whom they served as an evolutionary stepping stone to multicellularity.
Type I collagen is the most abundant collagen of the human body, consisting of around 90% of the body's total collagen in vertebrates. Due to this, it is also the most abundant protein type found in all vertebrates. Type I forms large, eosinophilic fibers known as collagen fibers, which make up most of the rope-like dense connective tissue in the body.

Tenascin X (TN-X), also known as flexillin or hexabrachion-like protein, is a 450kDa glycoprotein, a member of the tenascin family, that is expressed in connective tissues. In humans it is encoded by the TNXB gene.

Collagen alpha-1(V) chain is a protein that in humans is encoded by the COL5A1 gene.

Collagen alpha-2(V) chain is a protein that in humans is encoded by the COL5A2 gene.

Collagen alpha-2(I) chain is a protein that in humans is encoded by the COL1A2 gene.

Collagen alpha-1(XII) chain is a protein that in humans is encoded by the COL12A1 gene.

Collagen alpha-3(V) chain is a protein that in humans is encoded by the COL5A3 gene.
Collagen VI (ColVI) is a type of collagen primarily associated with the extracellular matrix of skeletal muscle. ColVI maintains regularity in muscle function and stabilizes the cell membrane. It is synthesized by a complex, multistep pathway that leads to the formation of a unique network of linked microfilaments located in the extracellular matrix (ECM). ColVI plays a vital role in numerous cell types, including chondrocytes, neurons, myocytes, fibroblasts, and cardiomyocytes. ColVI molecules are made up of three alpha chains: α1(VI), α2(VI), and α3(VI). It is encoded by 6 genes: COL6A1, COL6A2, COL6A3, COL6A4, COL6A5, and COL6A6. The chain lengths of α1(VI) and α2(VI) are about 1,000 amino acids. The chain length of α3(VI) is roughly a third larger than those of α1(VI) and α2(VI), and it consists of several spliced variants within the range of 2,500 to 3,100 amino acids.
Feline cutaneous asthenia is a rare inheritable skin disease of cats characterised by abnormal elasticity, stretching, and improper healing of the skin. Pendulous wing-like folds of skin form on the cat's back, shoulders and haunches. Even stroking the cat can cause the skin to stretch and tear. A recessive autosomal form of feline cutaneous asthenia has been identified in Siamese cats and related breeds. In the homozygous state, it is apparently lethal. Feline cutaneous asthenia is similar to the Ehlers–Danlos syndrome of humans.
FKBP14 is a gene which codes for a structural protein named FKBP prolyl isomerase 14. This protein is believed to aid in the process of procollagen folding and is located in the endoplasmic reticulum that functions to process and transport proteins. Procollagens are collagen precursors located in the extracellular matrix that give tissues elasticity, strength, and support. This gene is involved in patterning the collagen structure. FKBP prolyl isomerase 14 may also be involved in altering other factors in the extracellular matrix. Mutations of this gene are associated with the kyphoscoliotic type of Ehlers-Danlos syndrome. This condition is characterized by a high range of joint movement, muscle atrophy, curved spine, and delicate cardiovascular vessels. These symptoms are brought about by a loss of the protein which results in a disruption of endoplasmic reticulum activities and extracellular matrix organization. FKBP14 mRNA levels are found higher in ovarian cancer tissues than healthy ovarian tissue and knocked down expression of FKBP14 by lentiviral shRNA leads to an impaired proliferative ability of ovarian cancer cells.

Daniel S. Greenspan is an American biomedical scientist, academic and researcher. He is Kellett professor of Cell and Regenerative Biology at the University of Wisconsin-Madison School of Medicine and Public Health. He has authored over 120 publications. His research has mainly focused on genes encoding proteins of the extracellular space and possible links between defects in such genes and human development and disease.
References
- ↑ Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE (December 2004). "Type V collagen controls the initiation of collagen fibril assembly". The Journal of Biological Chemistry. 279 (51): 53331–53337. doi: 10.1074/jbc.M409622200 . PMID 15383546.
- ↑ Malfait F, Wenstrup R, De Paepe A (1993). Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A (eds.). "Ehlers-Danlos Syndrome, Classic Type". GeneReviews. Seattle (WA): University of Washington, Seattle. PMID 20301422.
- 1 2 3 4 "COL5A1 gene: MedlinePlus Genetics". medlineplus.gov. Retrieved 2023-04-26.
- 1 2 Martin, Patricia; Teodoro, Walcy R.; Velosa, Ana Paula P.; de Morais, Jymenez; Carrasco, Solange; Christmann, Romy B.; Goldenstein-Schainberg, Cláudia; Parra, Edwin R.; Katayama, Maria Lúcia; Sotto, Mirian N.; Capelozzi, Vera L.; Yoshinari, Natalino H. (September 2012). "Abnormal collagen V deposition in dermis correlates with skin thickening and disease activity in systemic sclerosis". Autoimmunity Reviews. 11 (11): 827–835. doi:10.1016/j.autrev.2012.02.017. ISSN 1873-0183. PMID 22406224.
- 1 2 3 4 5 6 7 8 9 10 11 Mak, Ki M.; Png, Chien Yi M.; Lee, Danielle J. (May 2016). "Type V Collagen in Health, Disease, and Fibrosis". Anatomical Record. 299 (5): 613–629. doi: 10.1002/ar.23330 . ISSN 1932-8494. PMID 26910848.
- ↑ "Studies on Collagen". www.collagencomplete.com. Retrieved 2016-07-13.
- ↑ Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, et al. (August 2002). "Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection". Journal of Immunology. 169 (3): 1542–1549. doi: 10.4049/jimmunol.169.3.1542 . PMID 12133982.
- ↑ Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Chiyo M, Lee J, et al. (October 2008). "Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction". Journal of Immunology. 181 (8): 5738–5747. doi:10.4049/jimmunol.181.8.5738. PMC 2997998 . PMID 18832733.