Related Research Articles
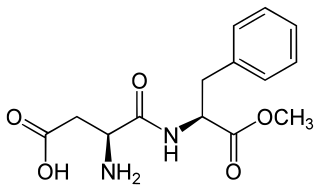
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose and is commonly used as a sugar substitute in foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide with brand names NutraSweet, Equal, and Canderel. Aspartame was approved by the US Food and Drug Administration (FDA) in 1974, and then again in 1981, after approval was revoked in 1980.

Food coloring, color additive or colorant is any dye, pigment, or substance that imparts color when it is added to food or beverages. Colorants can be supplied as liquids, powders, gels, or pastes. Food coloring is commonly used in commercial products and in domestic cooking.

The Food Standards Agency is a non-ministerial government department of the Government of the United Kingdom. It is responsible for protecting public health in relation to food in England, Wales and Northern Ireland. It is led by a board appointed to act in the public interest. Its headquarters are in London, with offices in York, Birmingham, Wales and Northern Ireland. Its counterpart in Scotland is Food Standards Scotland.

The Codex Alimentarius is a collection of internationally recognized standards, codes of practice, guidelines, and other recommendations published by the Food and Agriculture Organization of the United Nations relating to food, food production, food labeling, and food safety.
A novel food is a type of food that does not have a significant history of consumption or is produced by a method that has not previously been used for food.
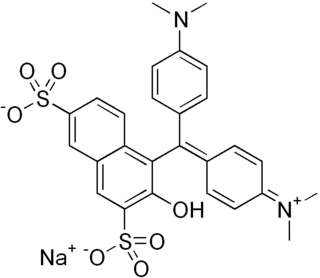
Green S is a green synthetic coal tar triarylmethane dye with the molecular formula C27H25N2O7S2Na.

The European Food Safety Authority (EFSA) is the agency of the European Union (EU) that provides independent scientific advice and communicates on existing and emerging risks associated with the food chain. EFSA was established in February 2002, is based in Parma, Italy, and for 2021 it has a budget of €118.6 million, and a total staff of 542.
Phytogenics are a group of natural growth promoters or non-antibiotic growth promoters used as feed additives, derived from herbs, spices or other plants. The term phytogenic feed additives was coined by an Austrian multinational feed additives company named Delacon, and was first introduced to the market in the 1980s.
The artificial sweetener aspartame has been the subject of several controversies since its initial approval by the U.S. Food and Drug Administration (FDA) in 1974. The FDA approval of aspartame was highly contested, beginning with suspicions of its involvement in brain cancer, alleging that the quality of the initial research supporting its safety was inadequate and flawed, and that conflicts of interest marred the 1981 approval of aspartame, previously evaluated by two FDA panels that concluded to keep the approval on hold before further investigation. In 1987, the U.S. Government Accountability Office concluded that the food additive approval process had been followed properly for aspartame. The irregularities fueled a conspiracy theory, which the "Nancy Markle" email hoax circulated, along with claims—counter to the weight of medical evidence—that numerous health conditions are caused by the consumption of aspartame in normal doses.

Food safety is used as a scientific method/discipline describing handling, preparation, and storage of food in ways that prevent foodborne illness. The occurrence of two or more cases of a similar illness resulting from the ingestion of a common food is known as a food-borne disease outbreak. This includes a number of routines that should be followed to avoid potential health hazards. In this way, food safety often overlaps with food defense to prevent harm to consumers. The tracks within this line of thought are safety between industry and the market and then between the market and the consumer. In considering industry-to-market practices, food safety considerations include the origins of food including the practices relating to food labeling, food hygiene, food additives and pesticide residues, as well as policies on biotechnology and food and guidelines for the management of governmental import and export inspection and certification systems for foods. In considering market-to-consumer practices, the usual thought is that food ought to be safe in the market and the concern is safe delivery and preparation of the food for the consumer. Food safety, nutrition and food security are closely related. Unhealthy food creates a cycle of disease and malnutrition that affects infants and adults as well.

Food safety in China is a widespread concern for the country's agricultural industry and consumers. China's principal crops are rice, corn, wheat, soybeans, and cotton in addition to apples and other fruits and vegetables. China's principal livestock products include pork, beef, dairy, and eggs. The Chinese government oversees agricultural production as well as the manufacture of food packaging, containers, chemical additives, drug production, and business regulation. In recent years, the Chinese government attempted to consolidate food safety regulation with the creation of the State Food and Drug Administration of China in 2003; officials have also been under increasing public and international pressure to solve food safety problems. Chinese Vice Premier Li Keqiang said, "Food is essential, and safety should be a top priority. Food safety is closely related to people's lives and health and economic development and social harmony," at a State Council meeting in Beijing.
The Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) is one of the independent scientific committees managed by the Directorate-General for Health and Consumer Protection of the European Commission, which provide scientific advice to the Commission on issues related to consumer products.
The Scientific Committee on Consumer Safety (SCCS) is one of the independent scientific committees managed by the Directorate-General for Health and Consumer Protection of the European Commission, which provide scientific advice to the commission on issues related to non-food issues. It is the successor to both the Scientific Committee on Consumer Products (SCCP) and the Scientific Committee on Cosmetic Products and Non-Food Products (SCCNFP).
The Scientific Committee on Health and Environmental Risks (SCHER) is one of the independent scientific committees managed by the Directorate-General for Health and Consumer Protection of the European Commission, which provide scientific advice to the Commission on issues related to consumer products.
The Directorate-General for Health and Food Safety, until 2014 known as the Directorate-General for Health and Consumers, is a directorate-general of the European Commission. The DG is responsible for the monitoring and implementation of European Union policies and laws on health and food safety. It is headed by European Commissioner for Health and Food Safety Stella Kyriakides and Director-General Sandra Gallina.

The Joint FAO/WHO Expert Committee on Food Additives (JECFA) is an international scientific expert committee that is administered jointly by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO). It has been meeting since 1956 to provide independent scientific advice pertaining to the safety evaluation of food additives. Its current scope of work now also includes the evaluation of contaminants, naturally occurring toxicants and residues of veterinary drugs in food.
The Hungarian Food Safety Office (HFSO) operated as the Hungarian partner institution of the European Food Safety Authority (EFSA) from 2003 to 2012 in conformity with the EU requirements. One of its priority was to assess the health risks derived from food and indirectly from feed, to liaise with international and Hungarian authorities, and to communicate with the public on food safety issues. From 2012, these tasks are performed by the National Food Chain Safety Office, which was established by the integration of the Central Agricultural Office and HFSO.
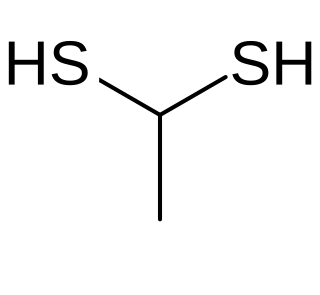
Ethane-1,1-dithiol is an organosulfur compound with formula CH3CH(SH)2. It is a colourless smelly liquid that is added to or found in some foods. The compound is an example of a geminal dithiol.

The Norwegian Scientific Committee for Food and Environment (VKM),, conducts open, independent, scientific risk assessments regarding safe food, food production and the environment upon request of the Norwegian Food Safety Authority and the Norwegian Environment Agency. VKM also carries out risk assessments on animal health, plant health, animal welfare and cosmetics for the Norwegian Food Safety Authority, and on microbiological products and alien organisms and trade in endangered species (CITES) upon request from the Norwegian Environment Agency. In addition, the Committee makes environmental risk assessments of genetically modified organisms for the Norwegian Environment Agency. The assessments are used as a basis for knowledge of the various ministries in Norway. The assessments also provide a basis for knowledge which the Norwegian Food Safety Authority uses to provide advice and determine regulations. The committee may raise matters on its own initiative. Risk assessments are made on an independent basis in accordance with internationally established methods. The scientific committee itself is to carry out risk assessments on their own initiative. The risk assessments are used by the Norwegian Food Safety Authority and the Norwegian Environment Agency when giving advice to Norwegian ministries, and when the Norwegian Food Safety Authority is developing new laws and regulations.

The German Federal Institute for Risk Assessment, abbreviated BfR, is a body under public law of the German federal government with full legal capacity. The institute comes under the portfolio of the Federal Ministry of Food and Agriculture and has the task of providing scientific advice to the federal government on issues relating to food safety, product safety, chemical safety, contaminants in the food chain, animal protection and consumer health protection. Further technical supervision is performed by the Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety and the Federal Ministry of Transport.