Related Research Articles

Leukemia, also spelled leukaemia, is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called blasts or leukemia cells. Symptoms may include bleeding and bruising, fatigue, fever, and an increased risk of infections. These symptoms occur due to a lack of normal blood cells. Diagnosis is typically made by blood tests or bone marrow biopsy.

Chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes. Early on there are typically no symptoms. Later non-painful lymph node swelling, feeling tired, fever, night sweats, or weight loss for no clear reason may occur. Enlargement of the spleen and low red blood cells (anemia) may also occur. It typically worsens gradually over years.

Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood. It may be autologous, allogeneic or syngeneic.

Acute lymphoblastic leukemia (ALL) is a cancer of the lymphoid line of blood cells characterized by the development of large numbers of immature lymphocytes. Symptoms may include feeling tired, pale skin color, fever, easy bleeding or bruising, enlarged lymph nodes, or bone pain. As an acute leukemia, ALL progresses rapidly and is typically fatal within weeks or months if left untreated.

A myeloid sarcoma, is a solid tumor composed of immature white blood cells called myeloblasts. A chloroma is an extramedullary manifestation of acute myeloid leukemia; in other words, it is a solid collection of leukemic cells occurring outside of the bone marrow.

Cyclophosphamide (CP), also known as cytophosphane among other names, is a medication used as chemotherapy and to suppress the immune system. As chemotherapy it is used to treat lymphoma, multiple myeloma, leukemia, ovarian cancer, breast cancer, small cell lung cancer, neuroblastoma, and sarcoma. As an immune suppressor it is used in nephrotic syndrome, granulomatosis with polyangiitis, and following organ transplant, among other conditions. It is taken by mouth or injection into a vein.
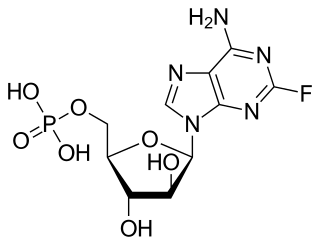
Fludarabine, sold under the brand name Fludara among others, is a chemotherapy medication used in the treatment of leukemia and lymphoma. These include chronic lymphocytic leukemia, non-Hodgkin's lymphoma, acute myeloid leukemia, and acute lymphocytic leukemia. It is given by injection into a vein or by mouth.
Gemtuzumab ozogamicin, sold under the brand name Mylotarg, is an antibody-drug conjugate that is used to treat acute myeloid leukemia.

Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production. Symptoms may include feeling tired, shortness of breath, easy bruising and bleeding, and increased risk of infection. Occasionally, spread may occur to the brain, skin, or gums. As an acute leukemia, AML progresses rapidly and is typically fatal within weeks or months if left untreated.

Cluster of differentiation antigen 135 (CD135) also known as fms like tyrosine kinase 3 (FLT-3), receptor-type tyrosine-protein kinase FLT3, or fetal liver kinase-2 (Flk2) is a protein that in humans is encoded by the FLT3 gene. FLT3 is a cytokine receptor which belongs to the receptor tyrosine kinase class III. CD135 is the receptor for the cytokine Flt3 ligand (FLT3L).
Juvenile myelomonocytic leukemia (JMML) is a serious chronic leukemia that affects children mostly aged 4 and younger. The name JMML now encompasses all diagnoses formerly referred to as juvenile chronic myeloid leukemia (JCML), chronic myelomonocytic leukemia of infancy, and infantile monosomy 7 syndrome. The average age of patients at diagnosis is 2 years old. The World Health Organization has included JMML in the category of myelodysplastic and myeloproliferative disorders.

Acute megakaryoblastic leukemia (AMKL) is life-threatening leukemia in which malignant megakaryoblasts proliferate abnormally and injure various tissues. Megakaryoblasts are the most immature precursor cells in a platelet-forming lineage; they mature to promegakaryocytes and, ultimately, megakaryocytes which cells shed membrane-enclosed particles, i.e. platelets, into the circulation. Platelets are critical for the normal clotting of blood. While malignant megakaryoblasts usually are the predominant proliferating and tissue-damaging cells, their similarly malignant descendants, promegakaryocytes and megakaryocytes, are variable contributors to the malignancy.
Biphenotypic acute leukaemia (BAL) is an uncommon type of leukemia which arises in multipotent progenitor cells which have the ability to differentiate into both myeloid and lymphoid lineages. It is a subtype of "leukemia of ambiguous lineage".
Acute panmyelosis with myelofibrosis (APMF) it is a poorly defined disorder that arises as either a clonal disorder, or following toxic exposure to the bone marrow.

Midostaurin, sold under the brand name Rydapt, is a multi-targeted protein kinase inhibitor that has been investigated for the treatment of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and advanced systemic mastocytosis. It is a semi-synthetic derivative of staurosporine, an alkaloid from the bacterium Streptomyces staurosporeus.
Graft-versus-tumor effect (GvT) appears after allogeneic hematopoietic stem cell transplantation (HSCT). The graft contains donor T cells that can be beneficial for the recipient by eliminating residual malignant cells. GvT might develop after recognizing tumor-specific or recipient-specific alloantigens. It could lead to remission or immune control of hematologic malignancies. This effect applies in myeloma and lymphoid leukemias, lymphoma, multiple myeloma and possibly breast cancer. It is closely linked with graft-versus-host disease (GvHD), as the underlying principle of alloimmunity is the same. CD4+CD25+ regulatory T cells (Treg) can be used to suppress GvHD without loss of beneficial GvT effect. The biology of GvT response still isn't fully understood but it is probable that the reaction with polymorphic minor histocompatibility antigens expressed either specifically on hematopoietic cells or more widely on a number of tissue cells or tumor-associated antigens is involved. This response is mediated largely by cytotoxic T lymphocytes (CTL) but it can be employed by natural killers as separate effectors, particularly in T-cell-depleted HLA-haploidentical HSCT.
"7+3" in the context of chemotherapy is an acronym for a chemotherapy regimen that is most often used today as first-line induction therapy in acute myelogenous leukemia, excluding the acute promyelocytic leukemia form, which is better treated with ATRA and/or arsenic trioxide and requires less chemotherapy.
ADE is a chemotherapy regimen most often used as an induction or consolidation regimen in acute myelogenous leukemia, especially in poor-risk patients or those refractory to the standard first-line induction with standard "7+3" regimen or who are relapsed after the standard chemotherapy.
Guo Mei is a hematologist and associate director of 307th Hospital of Chinese People’s Liberation Army and deputy director of Radiation Research Institute.
Camidanlumab tesirine is an antibody-drug conjugate (ADC) composed of a human antibody that binds to the protein CD25, conjugated to a pyrrolobenzodiazepine dimer toxin. The experimental drug, developed by ADC Therapeutics is being tested in clinical trials for the treatment of B-cell Hodgkin's lymphoma (HL) and non-Hodgkin lymphoma (NHL), and for the treatment of B-cell acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).
References
- ↑ Visani G, Tosi P, Zinzani PL, et al. (November 1994). "FLAG (fludarabine + high-dose cytarabine + G-CSF): an effective and tolerable protocol for the treatment of 'poor risk' acute myeloid leukemias". Leukemia. 8 (11): 1842–6. PMID 7526088.
- ↑ Pastore D, Specchia G, Carluccio P, et al. (April 2003). "FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: single-center experience". Annals of Hematology. 82 (4): 231–5. doi:10.1007/s00277-003-0624-2. PMID 12707726.
- ↑ Jackson GH (2004). "Use of fludarabine in the treatment of acute myeloid leukemia". The Hematology Journal. 5 Suppl 1: S62–7. doi:10.1038/sj.thj.6200392. PMID 15079154.
- ↑ Specchia G, Pastore D, Carluccio P, et al. (November 2005). "FLAG-IDA in the treatment of refractory/relapsed adult acute lymphoblastic leukemia". Annals of Hematology. 84 (12): 792–5. doi:10.1007/s00277-005-1090-9. PMID 16047203.
- ↑ Luo S, Cai F, Jiang L, et al. (March 2013). "Clinical study of Mito-FLAG regimen in treatment of relapsed acute myeloid leukemia". Experimental and Therapeutic Medicine. 5 (3): 982–986. doi:10.3892/etm.2013.917. PMC 3570250 . PMID 23407597.
- ↑ Hänel M, Friedrichsen K, Hänel A, et al. (August 2001). "Mito-flag as salvage therapy for relapsed and refractory acute myeloid leukemia". Onkologie. 24 (4): 356–60. doi:10.1159/000055107. PMID 11574763.
- ↑ Saure C, Schroeder T, Zohren F, et al. (March 2012). "Upfront allogeneic blood stem cell transplantation for patients with high-risk myelodysplastic syndrome or secondary acute myeloid leukemia using a FLAMSA-based high-dose sequential conditioning regimen". Biology of Blood and Marrow Transplantation. 18 (3): 466–72. doi: 10.1016/j.bbmt.2011.09.006 . PMID 21963618.
- ↑ Chemnitz JM, von Lilienfeld-Toal M, Holtick U, et al. (January 2012). "Intermediate intensity conditioning regimen containing FLAMSA, treosulfan, cyclophosphamide, and ATG for allogeneic stem cell transplantation in elderly patients with relapsed or high-risk acute myeloid leukemia". Annals of Hematology. 91 (1): 47–55. doi:10.1007/s00277-011-1253-9. PMID 21584670.
- ↑ Krejci M, Doubek M, Dusek J, et al. (October 2013). "Combination of fludarabine, amsacrine, and cytarabine followed by reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation in patients with high-risk acute myeloid leukemia". Annals of Hematology. 92 (10): 1397–403. doi:10.1007/s00277-013-1790-5. PMID 23728608.
- ↑ Boehm A, Rabitsch W, Locker GJ, et al. (June 2011). "Successful allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia during respiratory failure and invasive mechanical ventilation". Wiener Klinische Wochenschrift. 123 (11–12): 354–8. doi:10.1007/s00508-011-1590-7. PMID 21633813.
- ↑ Schmid C, Schleuning M, Tischer J, et al. (January 2012). "Early allo-SCT for AML with a complex aberrant karyotype—results from a prospective pilot study". Bone Marrow Transplantation. 47 (1): 46–53. doi: 10.1038/bmt.2011.15 . PMID 21358688.
- ↑ Zohren F, Czibere A, Bruns I, et al. (December 2009). "Fludarabine, amsacrine, high-dose cytarabine and 12 Gy total body irradiation followed by allogeneic hematopoietic stem cell transplantation is effective in patients with relapsed or high-risk acute lymphoblastic leukemia". Bone Marrow Transplantation. 44 (12): 785–92. doi: 10.1038/bmt.2009.83 . PMID 19430496.
- ↑ Schmid C, Weisser M, Ledderose G, Stötzer O, Schleuning M, Kolb HJ (October 2002). "[Dose-reduced conditioning before allogeneic stem cell transplantation: principles, clinical protocols and preliminary results]". Deutsche Medizinische Wochenschrift (in German). 127 (42): 2186–92. doi:10.1055/s-2002-34946. PMID 12397547.