Related Research Articles

Prostate cancer is the uncontrolled growth of cells in the prostate, a gland in the male reproductive system below the bladder. Early prostate cancer usually causes no symptoms. As the tumor grows, it can damage nearby organs causing erectile dysfunction, blood in the urine or semen, and trouble urinating. Some tumors eventually spread to other areas of the body, particularly the bones and lymph nodes. There, tumors cause severe bone pain, leg weakness or paralysis, and eventually death.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.

DU145 (DU-145) is a human prostate cancer cell line. DU145, PC3, and LNCaP are considered to be the standard prostate cancer cell lines used in therapeutic research.
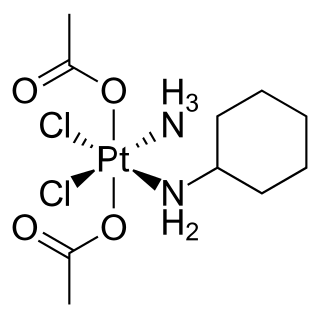
Satraplatin is a platinum-based antineoplastic agent that was under investigation as a treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues—cisplatin, carboplatin, and oxaliplatin—must be given intravenously.
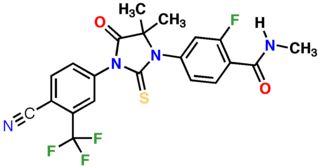
Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
Radium-223 is an isotope of radium with an 11.4-day half-life. It was discovered in 1905 by T. Godlewski, a Polish chemist from Kraków, and was historically known as actinium X (AcX). Radium-223 dichloride is an alpha particle-emitting radiotherapy drug that mimics calcium and forms complexes with hydroxyapatite at areas of increased bone turnover. The principal use of radium-223, as a radiopharmaceutical to treat metastatic cancers in bone, takes advantage of its chemical similarity to calcium, and the short range of the alpha radiation it emits.
James L. Gulley is an American cancer researcher and the Director of the Medical Oncology Service at National Cancer Institute.
William K. Oh, is an American medical oncologist, academic and industry leader and expert in the management of genitourinary malignancies, including prostate, renal, bladder and testicular cancers.
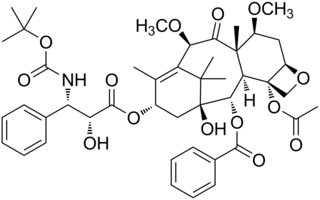
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.
Sipuleucel-T, sold under the brand name Provenge, developed by Dendreon Pharmaceuticals, LLC, is a cell-based cancer immunotherapy for prostate cancer (CaP). It is an autologous cellular immunotherapy.

Tasquinimod is an experimental drug currently being investigated for the treatment of solid tumors. Tasquinimod has been mostly studied in prostate cancer, but its mechanism of action suggests that it could be used to treat other cancers. Castration-resistant prostate cancer (CRPC), formerly called hormone-resistant or hormone-refractory prostate cancer, is prostate cancer that grows despite medical or surgical androgen deprivation therapy. Tasquinimod targets the tumor microenvironment and counteracts cancer development by inhibiting angiogenesis and metastasis and by modulating the immune system. It is now in phase III development, following successful phase II trial outcomes.
Eftilagimod alpha is a large-molecule cancer drug being developed by the clinical-stage biotechnology company Immutep. Efti is a soluble version of the immune checkpoint molecule LAG-3. It is an APC Activator used to increase an immune response to tumors, and is administered by subcutaneous injection. Efti has three intended clinical settings:
Darolutamide, sold under the brand name Nubeqa, is an antiandrogen medication which is used in the treatment of non-metastatic castration-resistant prostate cancer in men. It is specifically approved to treat non-metastatic castration-resistant prostate cancer (nmCRPC) in conjunction with surgical or medical castration. The medication is taken by mouth twice per day with food.
Abituzumab is a humanized IgG2 monoclonal antibody (mAb) targeted at CD51 currently in development by Merck KGaA Darmstadt, Germany in an attempt to prevent bone lesion metastases in castration-resistant prostate cancer.

Ralaniten acetate is a first-in-class antiandrogen that targets the N-terminal domain (NTD) of the androgen receptor (AR) developed by ESSA Pharmaceuticals and was under investigation for the treatment of prostate cancer. This mechanism of action is believed to allow the drug to block signaling from the AR and its splice variants. EPI-506 is a derivative of bisphenol A and a prodrug of ralaniten (EPI-002), one of the four stereoisomers of EPI-001, and was developed as a successor of EPI-001. The drug reached phase I/II prior to the discontinuation of its development. It showed signs of efficacy in the form of prostatic specific antigen (PSA) decreases (4–29%) predominantly at higher doses (≥1,280 mg) in some patients but also caused side effects and was discontinued by its developer in favor of next-generation AR NTD inhibitors with improved potency and tolerability.
The dendritic cell-based cancer vaccine is an innovation in therapeutic strategy for cancer patients.
A therapeutic vaccine is a vaccine which is administered after a disease or infection has already occurred. A therapeutic vaccine works by activating the immune system of a patient to fight an infection. A therapeutic vaccine differs from a prophylactic vaccine in that prophylactic vaccines are administered to individuals as a precautionary measure to avoid the infection or disease while therapeutic vaccines are administered after the individual is already affected by the disease or infection. A therapeutic vaccine fights an existing infection in the body rather than immunizing the body for protection against future diseases and infections. Therapeutic vaccines are mostly used against viral infections. Patients affected with chronic viral infections are administered with therapeutic vaccines, as their immune system is not able to produce enough efficient antibodies.

Lutetium (177Lu) vipivotide tetraxetan, sold under the brand name Pluvicto, is a radiopharmaceutical medication used for the treatment of prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC). Lutetium (177Lu) vipivotide tetraxetan is a targeted radioligand therapy.
References
- ↑ "Survival Benefit Propels Prostate Cancer Vaccine to Phase III Trial". OncLive. June 2013. 2 (3). 3 July 2013. Retrieved 2014-10-31.
- ↑ "A Randomized, Double-blind, Phase 3 Efficacy Trial of PROSTVAC-V/F +/- GM-CSF in Men With Asymptomatic or Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer (Prospect)". Clinicaltrials.gov. Retrieved 31 October 2014.
- ↑ Revised Overall Survival Analysis of a Phase II, Randomized, Double-Blind, Controlled Study of PROSTVAC in Men With Metastatic Castration-Resistant Prostate Cancer Philip W. Kantoff, James L. Gulley, and Cesar Pico-Navarro Journal of Clinical Oncology 2017 35:1, 124-125
- ↑ Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR (2010). "Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer". J Clin Oncol. 28 (7): 1099–105. doi:10.1200/JCO.2009.25.0597. PMC 2834462 . PMID 20100959.
- ↑ Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, Dahut WL, Schlom J, Gulley JL (2012). "Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial". The Lancet Oncology. 13 (5): 501–8. doi:10.1016/S1470-2045(12)70006-2. PMC 6359905 . PMID 22326924.
- ↑ Jochems C, Tucker JA, Tsang KY, Madan RA, Dahut WL, Liewehr DJ, Steinberg SM, Gulley JL, Schlom J (2014). "A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates". Cancer Immunol Immunother. 63 (4): 407–18. doi:10.1007/s00262-014-1524-0. PMC 6314199 . PMID 24514956.
- 1 2 Staff (1 April 2015). "Bavarian Nordic Could Reap $975M in Prostate Cancer Deal with BMS". Genetic Engineering & Biotechnology News (Paper). 35 (7): 12. Retrieved 2016-06-12.