In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as cancer cells, parasitic worms, and also objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions.

A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.
An epitope, also known as antigenic determinant, is the part of an antigen that is recognized by the immune system, specifically by antibodies, B cells, or T cells. The part of an antibody that binds to the epitope is called a paratope. Although epitopes are usually non-self proteins, sequences derived from the host that can be recognized are also epitopes.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

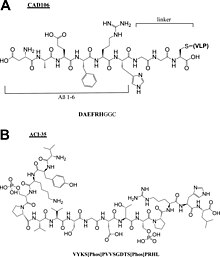
A conjugate vaccine is a type of subunit vaccine which combines a weak antigen with a strong antigen as a carrier so that the immune system has a stronger response to the weak antigen.
In academia, computational immunology is a field of science that encompasses high-throughput genomic and bioinformatics approaches to immunology. The field's main aim is to convert immunological data into computational problems, solve these problems using mathematical and computational approaches and then convert these results into immunologically meaningful interpretations.
Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted:
rBCG30 is a prospective Bacillus Calmette-Guérin vaccine against tuberculosis. It is a live vaccine, consisting of BCG, which has been evaluated as a tuberculosis vaccination. It is genetically modified to produce abundant amounts of mycolyl transferase, a 30kDa antigen that has been shown to produce a strong immune response in animals and humans. rBCG30 had been in human clinical trials, but no clinical development has been reported since 2007.
Molecular mimicry is the theoretical possibility that sequence similarities between foreign and self-peptides are enough to result in the cross-activation of autoreactive T or B cells by pathogen-derived peptides. Despite the prevalence of several peptide sequences which can be both foreign and self in nature, just a few crucial residues can activate a single antibody or TCR. This highlights the importance of structural homology in the theory of molecular mimicry. Upon activation, these "peptide mimic" specific T or B cells can cross-react with self-epitopes, thus leading to tissue pathology (autoimmunity). Molecular mimicry is one of several ways in which autoimmunity can be evoked. A molecular mimicking event is more than an epiphenomenon despite its low probability, and these events have serious implications in the onset of many human autoimmune disorders.
Cancer/testis antigen 1 also known as LAGE2 or LAGE2B is a protein that in humans is encoded by the CTAG1B gene. It is most often referenced by its alias NY-ESO-1.

Keyhole limpet hemocyanin (KLH) is a large, multisubunit, oxygen-carrying, metalloprotein that is found in the hemolymph of the giant keyhole limpet, Megathura crenulata, a species of keyhole limpet that lives off the coast of California, from Monterey Bay to Isla Asuncion off Baja California.
A subunit vaccine is a vaccine that contains purified parts of the pathogen that are antigenic, or necessary to elicit a protective immune response. Subunit vaccine can be made from dissembled viral particles in cell culture or recombinant DNA expression, in which case it is a recombinant subunit vaccine.
Active immunotherapy is a type of immunotherapy that aims to stimulate the host's immune system or a specific immune response to a disease or pathogen and is most commonly used in cancer treatments. Active immunotherapy is also used for treatment of neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, prion disease, and multiple sclerosis. Active immunotherapies induce an immune response through direct immune system stimulation, while immunotherapies that administer antibodies directly to the system are classified as passive immunotherapies. Active immunotherapies can elicit generic and specific immune responses depending on the goal of the treatment. The categories of active immunotherapy divide into:
Immunomics is the study of immune system regulation and response to pathogens using genome-wide approaches. With the rise of genomic and proteomic technologies, scientists have been able to visualize biological networks and infer interrelationships between genes and/or proteins; recently, these technologies have been used to help better understand how the immune system functions and how it is regulated. Two thirds of the genome is active in one or more immune cell types and less than 1% of genes are uniquely expressed in a given type of cell. Therefore, it is critical that the expression patterns of these immune cell types be deciphered in the context of a network, and not as an individual, so that their roles be correctly characterized and related to one another. Defects of the immune system such as autoimmune diseases, immunodeficiency, and malignancies can benefit from genomic insights on pathological processes. For example, analyzing the systematic variation of gene expression can relate these patterns with specific diseases and gene networks important for immune functions.
Immunodominance is the immunological phenomenon in which immune responses are mounted against only a few of the antigenic peptides out of the many produced. That is, despite multiple allelic variations of MHC molecules and multiple peptides presented on antigen presenting cells, the immune response is skewed to only specific combinations of the two. Immunodominance is evident for both antibody-mediated immunity and cell-mediated immunity. Epitopes that are not targeted or targeted to a lower degree during an immune response are known as subdominant epitopes. The impact of immunodominance is immunodomination, where immunodominant epitopes will curtail immune responses against non-dominant epitopes. Antigen-presenting cells such as dendritic cells, can have up to six different types of MHC molecules for antigen presentation. There is a potential for generation of hundreds to thousands of different peptides from the proteins of pathogens. Yet, the effector cell population that is reactive against the pathogen is dominated by cells that recognize only a certain class of MHC bound to only certain pathogen-derived peptides presented by that MHC class. Antigens from a particular pathogen can be of variable immunogenicity, with the antigen that stimulates the strongest response being the immunodominant one. The different levels of immunogenicity amongst antigens forms what is known as dominance hierarchy.
Artificial antigen presenting cells (aAPCs) are engineered platforms for T-cell activation. aAPCs are used as a new technology and approach to cancer immunotherapy. Immunotherapy aims to utilize the body's own defense mechanism—the immune system—to recognize mutated cancer cells and to kill them the way the immune system would recognize and kill a virus or other micro-organisms causing infectious diseases. Antigen presenting cells are the sentinels of the immune system and patrol the body for pathogens. When they encounter foreign pathogens, the antigen presenting cells activate the T cells—"the soldiers of the immune system"— by delivering stimulatory signals that alert there is foreign material in the body with specific cell surface molecules (epitopes). aAPCs are synthetic versions of these sentinel cells and are made by attaching the specific T-cell stimulating signals to various macro and micro biocompatible surfaces like micron-sized beads. This can potentially reduce the cost while allowing control over generating large numbers of functional pathogen-specific T cells for therapy. Activated and stimulated T cells can be studied in this biomimetic contex and used for adoptive transfer as an immunotherapy.
Cancer/testis (CT) antigens are a group of proteins united by their importance in development and in cancer immunotherapy. In general, expression of these proteins is restricted to male germ cells in the adult animal. However, in cancer these developmental antigens are often re-expressed and can serve as a locus of immune activation. Thus, they are often classified as tumor antigens. The expression of CT antigens in various malignancies is heterogeneous and often correlates with tumor progression. CT antigens have been described in melanoma, liver cancer, lung cancer, bladder cancer, and pediatric tumors such as neuroblastoma. Gametogenesis offers an important role for many of these antigens in the differentiation, migration, and cell division of primordial germ cells, spermatogonia spermatocytes and spermatids. Because of their tumor-restricted expression and strong in vivo immunogenicity, CT antigens are identified as ideal targets for tumor specific immunotherapeutic approaches and prompted the development of several clinical trials of CT antigens-based vaccine therapy. CT antigens have been found to have at least 70 families so far, including about 140 members, most of which are expressed during spermatogenesis. Their expression are mainly regulated by epigenetic events, specifically, DNA methylation.
Neoepitopes are a class of major histocompatibility complex (MHC) bounded peptides. They represent the antigenic determinants of neoantigens. Neoepitopes are recognized by the immune system as targets for T cells and can elicit immune response to cancer.

UB-612 is a COVID-19 vaccine candidate developed by United Biomedical Asia, and Vaxxinity, Inc. It is a peptide vaccine.