 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fleet, Dulcolax, Brooklax, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601027 |
| License data | |
| Routes of administration | By mouth, rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 15%[ citation needed ] |
| Metabolism | Liver (CYP450-mediated) |
| Elimination half-life | 16 hours[ citation needed ] |
| Excretion | Primarily in the feces, systemically absorbed drug is excreted in the urine[ citation needed ] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.009.132 |
| Chemical and physical data | |
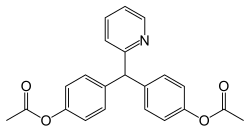
| Formula | C22H19NO4 |
| Molar mass | 361.397 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bisacodyl is an organic compound that is used as a stimulant laxative drug. It works directly on the colon to produce a bowel movement. It is typically prescribed for relief of episodic and chronic constipation and for the management of neurogenic bowel dysfunction, as well as part of bowel preparation before medical examinations, such as for a colonoscopy. [2] [3]
Contents
Bisacodyl is a derivative of triphenylmethane. It was first used as a laxative in 1953 because of its structural similarity to phenolphthalein. [4] [5]
It is on the World Health Organization's List of Essential Medicines. [6] In 2023, it was the 293rd most commonly prescribed medication in the United States, with more than 400,000 prescriptions. [7] [8]