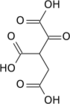
cis-aconitic acid | |
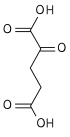
 trans-aconitic acid | |
| Names | |
|---|---|
| Preferred IUPAC name Prop-1-ene-1,2,3-tricarboxylic acid | |
| Other names Achilleic acid; equisetic acid; citridinic acid; pyrocitric acid; achilleaic acid; acinitic acid | |
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.007.162 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| Properties | |
| C6H6O6 | |
| Molar mass | 174.108 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 190 °C (374 °F; 463 K) (decomposes) (mixed isomers), 173 °C (cis and trans isomers) |
| Acidity (pKa) | 2.80, 4.46 (trans isomer) [2] 2.78, 4.41, 6.21 (cis isomer) [3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Aconitic acid refers to organic compounds with the formula HO2CCH2C(CO2H)=CHCO2H. A white solid, it is classified as a tricarboxylic acid. The two isomers are cis-aconitic acid and trans-aconitic acid. The conjugate base of cis-aconitic acid, cis-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase.
Aconitic acid can be synthesized by dehydration of citric acid using sulfuric acid: [4]
- (HO2CCH2)2C(OH)CO2H → HO2CCH=C(CO2H)CH2CO2H + H2O
A mixture of isomers is generated in this way.
Aconitic acid was originally isolated from Aconitum napellus by Swiss chemist and apothecary Jacques Peschier in 1820. [5] [6] It was first prepared by thermal dehydration. [7]
Like the conjugate bases of other polycarboxylic acid, acotinic acid forms a variety of coordination complexes. One example is the coordination polymer [Zn3(C6H3O6)2(H2O)6]n. [8]