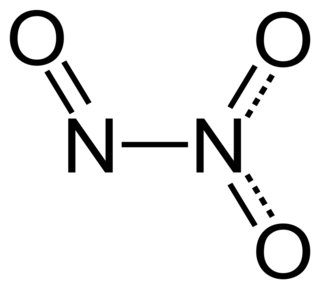
The nitronium ion, [NO2]+, is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion [NH4]+. It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule NO2, or the protonation of nitric acid HNO3.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that contain only nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents. As confirmed by X-ray crystallography, nitrobenzene is a planar molecule.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.
The nitrosonium ion is NO+, in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: NOClO4, NOSO4H (nitrosylsulfuric acid, more descriptively written ONSO3OH) and NOBF4. The ClO−4 and BF−4 salts are slightly soluble in acetonitrile CH3CN. NOBF4 can be purified by sublimation at 200–250 °C and 0.01 mmHg (1.3 Pa).

Tetrafluoroborate is the anion BF−
4. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF2−
4), tetrafluoromethane (CF4), and tetrafluoroammonium (NF+
4) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO−
4, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.

Triethyloxonium tetrafluoroborate is the organic oxonium compound with the formula [(CH3CH2)3O]+[BF4]−. It is often called Meerwein's reagent or Meerwein's salt after its discoverer Hans Meerwein. Also well known and commercially available is the related trimethyloxonium tetrafluoroborate. The compounds are white solids that dissolve in polar organic solvents. They are strong alkylating agents. Aside from the BF−4 salt, many related derivatives are available.
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen (OBE/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with water (H2O). During the synthesis, ammonium chloride is also produced.
Sodium tetrafluoroborate is an inorganic compound with formula NaBF4. It is a salt that forms colorless or white rhombic crystals and is soluble in water (108 g/100 mL) but less soluble in organic solvents.
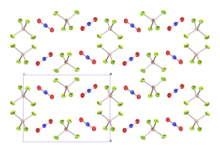
Dinitrogen trioxide is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):

Nitrosonium tetrafluoroborate, also called nitrosyl tetrafluoroborate, is a chemical compound with the chemical formula NOBF4. This colourless solid is used in organic synthesis as a nitrosating agent, diazotizing agent and a mild oxidant.

Copper(II) tetrafluoroborate is any inorganic compound with the formula Cu(H2O)x(BF4)2. As usually encountered, it is assumed to be the hexahydrate (x = 6), but this salt can be partially dehydrated to the tetrahydrate. Regardless, these compounds are aquo complexes of copper in its +2 oxidation state, with two weakly coordinating tetrafluoroborate anions.
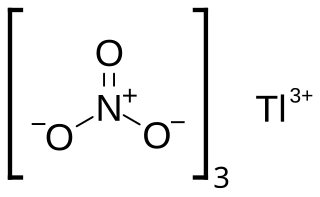
Thallium(III) nitrate, also known as thallic nitrate, is a thallium compound with chemical formula Tl(NO3)3. It is normally found as the trihydrate. It is a colorless and highly toxic salt. It is a strong oxidizing agent useful in organic synthesis. Among its many transformations, it oxidizes methoxyl phenols to quinone acetals, alkenes to acetals, and cyclic alkenes to ring-contracted aldehydes.

Diamantane is an organic compound that is a member of the diamondoids. These are cage hydrocarbons with structures similar to a subunit of the diamond lattice. It is a colorless solid that has been a topic of research since its discovery in oil and separation from deep natural gas condensates. Diamondoids such as diamantane exhibit unusual properties, including low surface energies, high densities, high hydrophobicities, and resistance to oxidation.
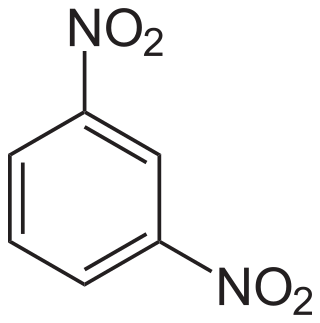
1,3-Dinitrobenzene is one of three isomers of dinitrobenzene, with the formula C6H4(NO2)2. It is one of three isomers of dinitrobenzene. The compound is a yellow solid that is soluble in organic solvents.

Zirconium nitrate is a volatile anhydrous transition metal nitrate salt of zirconium with formula Zr(NO3)4. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate.