
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin molybdaenum, which is based on Ancient Greek Μόλυβδος molybdos, meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".

Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 is α polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

Zinc sulfate describes a family of inorganic compounds with the formula ZnSO4(H2O)x. All are colorless solids. The most common form includes water of crystallization as the heptahydrate, with the formula ZnSO4·7H2O. It was historically known as "white vitriol". Zinc sulfate and its hydrates are colourless solids.

Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically.

Zinc sulfide is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics.

Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
In chemistry, a corrosion inhibitor or anti-corrosive is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy, that comes into contact with the fluid. The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime. Corrosion inhibitors are common in industry, and also found in over-the-counter products, typically in spray form in combination with a lubricant and sometimes a penetrating oil. They may be added to water to prevent leaching of lead or copper from pipes.
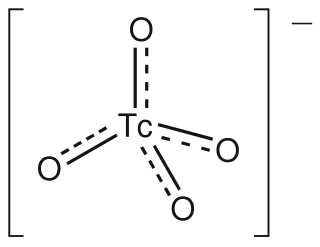
The pertechnetate ion is an oxyanion with the chemical formula TcO−
4. It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the 99mTc isotope which is commonly used in nuclear medicine in several nuclear scanning procedures.

Sodium molybdate, Na2MoO4, is useful as a source of molybdenum. This white, crystalline salt is often found as the dihydrate, Na2MoO4·2H2O.

Cadmium oxide is an inorganic compound with the formula CdO. It is one of the main precursors to other cadmium compounds. It crystallizes in a cubic rocksalt lattice like sodium chloride, with octahedral cation and anion centers. It occurs naturally as the rare mineral monteponite. Cadmium oxide can be found as a colorless amorphous powder or as brown or red crystals. Cadmium oxide is an n-type semiconductor with a band gap of 2.18 eV at room temperature.

Zinc chromate, ZnCrO4, is a chemical compound containing the chromate anion, appearing as odorless yellow powder or yellow-green crystals, but, when used for coatings, pigments are often added. It is used industrially in chromate conversion coatings, having been developed by the Ford Motor Company in the 1920s.

Sodium persulfate is the inorganic compound with the formula Na2S2O8. It is the sodium salt of peroxydisulfuric acid, H2S2O8, an oxidizing agent. It is a white solid that dissolves in water. It is almost non-hygroscopic and has good shelf-life.

Zinc nitride (Zn3N2) is an inorganic compound of zinc and nitrogen, usually obtained as (blue)grey crystals. It is a semiconductor. In pure form, it has the anti-bixbyite structure.
Zinc phosphate is an inorganic compound with the formula Zn3(PO4)2. This white powder is widely used as a corrosion resistant coating on metal surfaces either as part of an electroplating process or applied as a primer pigment (see also red lead). It has largely displaced toxic materials based on lead or chromium, and by 2006 it had become the most commonly used corrosion inhibitor. Zinc phosphate coats better on a crystalline structure than bare metal, so a seeding agent is often used as a pre-treatment. One common agent is sodium pyrophosphate.
Cobalt green is an ambiguous term for either of two families of green inorganic pigments. Both are obtained by doping cobalt(II) oxide into colorless host oxides.

Ammonium heptamolybdate is the inorganic compound whose chemical formula is (NH4)6Mo7O24, normally encountered as the tetrahydrate. A dihydrate is also known. It is a colorless solid, often referred to as ammonium paramolybdate or simply as ammonium molybdate, although "ammonium molybdate" can also refer to ammonium orthomolybdate, (NH4)2MoO4, and several other compounds. It is one of the more common molybdenum compounds.
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript behavior, they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.
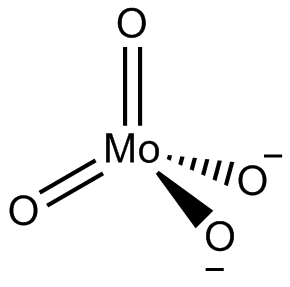
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxoanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxoanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.


















