
Molybdenum is a chemical element; it has symbol Mo and atomic number 42. The name derived from Ancient Greek Μόλυβδος molybdos, meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.

An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

In chemistry, a polyoxometalate is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form closed 3-dimensional frameworks. The metal atoms are usually group 6 or less commonly group 5 and group 7 transition metals in their high oxidation states. Polyoxometalates are often colorless, orange or red diamagnetic anions. Two broad families are recognized, isopolymetalates, composed of only one kind of metal and oxide, and heteropolymetalates, composed of one or more metals, oxide, and eventually a main group oxyanion. Many exceptions to these general statements exist.
Molybdenum trioxide describes a family of inorganic compounds with the formula MoO3(H2O)n where n = 0, 1, 2. The anhydrous compound is produced on the largest scale of any molybdenum compound since it is the main intermediate produced when molybdenum ores are purified. The anhydrous oxide is a precursor to molybdenum metal, an important alloying agent. It is also an important industrial catalyst. It is a yellow solid, although impure samples can appear blue or green.

Ammonium persulfate (APS) is the inorganic compound with the formula (NH4)2S2O8. It is a colourless (white) salt that is highly soluble in water, much more so than the related potassium salt. It is a strong oxidizing agent that is used as a catalyst in polymer chemistry, as an etchant, and as a cleaning and bleaching agent.

Sodium molybdate, Na2MoO4, is useful as a source of molybdenum. This white, crystalline salt is often encountered as the dihydrate, Na2MoO4·2H2O.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.

Ammonium tetrathiomolybdate is the chemical compound with the formula (NH4)2MoS4. This bright red ammonium salt is an important reagent in the chemistry of molybdenum and has been used as a building block in bioinorganic chemistry. The thiometallate (see metallate) anion has the distinctive property of undergoing oxidation at the sulfur centers concomitant with reduction of the metal from Mo(VI) to Mo(IV).

Ammonium paratungstate (or APT) is a white crystalline salt with the chemical formula (NH4)10(H2W12O42)·4H2O. It is described as "the most important raw material for all other tungsten products."

Ammonium heptamolybdate is the inorganic compound whose chemical formula is (NH4)6Mo7O24, normally encountered as the tetrahydrate. A dihydrate is also known. It is a colorless solid, often referred to as ammonium paramolybdate or simply as ammonium molybdate, although "ammonium molybdate" can also refer to ammonium orthomolybdate, (NH4)2MoO4, and several other compounds. It is one of the more common molybdenum compounds.

Molybdenum blue is a term applied to:
Aluminium molybdate is the chemical compound Al2(MoO4)3. It forms in certain hydrodesulfurization catalysts when alumina is doped with excess molybdenum. When molybdates are used to inhibit corrosion in aluminum piping, the protective film formed is hydrated aluminum molybdate. Although small quantities of aluminum molybdate form during aluminothermic reduction of molybdia, mechanical activation inhibits their formation.
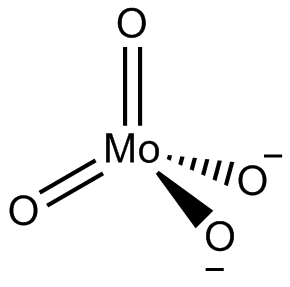
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of +6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.
Ammonium orthomolybdate is the inorganic compound with the chemical formula (NH4)2MoO4. It is a white solid that is prepared by treating molybdenum trioxide with aqueous ammonia. Upon heating these solutions, ammonia is lost, to give ammonium heptamolybdate ((NH4)6Mo7O24·4H2O).

Manganese(II) molybdate is an inorganic compound with the chemical formula MnMoO4. α-MnMoO4 has a monoclinic crystal structure. It is also antiferromagnetic at low temperatures.
Langbeinites are a family of crystalline substances based on the structure of langbeinite with general formula M2M'2(SO4)3, where M is a large univalent cation, and M' is a small divalent cation. The sulfate group, SO2−4, can be substituted by other tetrahedral anions with a double negative charge such as tetrafluoroberyllate, selenate, chromate, molybdate, or tungstates. Although monofluorophosphates are predicted, they have not been described. By redistributing charges other anions with the same shape such as phosphate also form langbeinite structures. In these the M' atom must have a greater charge to balance the extra three negative charges.
Scandium bromide, or ScBr3, is a trihalide, hygroscopic, water-soluble chemical compound of scandium and bromine.
Neodymium molybdate is an inorganic compound, with the chemical formula of Nd2(MoO4)3.

Molybdenum difluoride dioxide is the inorganic compound with the formula MoF2O2. It is a white, diamagnetic, volatile solid.












