
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.

Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. making it one of the last two stable elements to be discovered. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.

Zirconium is a chemical element; it has symbol Zr and atomic number 40. The name zirconium is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium.

Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is abundant as it comprises several minerals and synthetic products. All forms are white or colorless, although impure samples can be colored.

Zirconium dioxide, sometimes known as zirconia, is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.
In semiconductor manufacturing, a low-κ is a material with a small relative dielectric constant relative to silicon dioxide. Low-κ dielectric material implementation is one of several strategies used to allow continued scaling of microelectronic devices, colloquially referred to as extending Moore's law. In digital circuits, insulating dielectrics separate the conducting parts from one another. As components have scaled and transistors have gotten closer together, the insulating dielectrics have thinned to the point where charge build up and crosstalk adversely affect the performance of the device. Replacing the silicon dioxide with a low-κ dielectric of the same thickness reduces parasitic capacitance, enabling faster switching speeds and lower heat dissipation. In conversation such materials may be referred to as "low-k" rather than "low-κ" (low-kappa).

Titanium nitride is an extremely hard ceramic material, often used as a physical vapor deposition (PVD) coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface properties.
In the semiconductor industry, the term high-κ dielectric refers to a material with a high dielectric constant, as compared to silicon dioxide. High-κ dielectrics are used in semiconductor manufacturing processes where they are usually used to replace a silicon dioxide gate dielectric or another dielectric layer of a device. The implementation of high-κ gate dielectrics is one of several strategies developed to allow further miniaturization of microelectronic components, colloquially referred to as extending Moore's Law.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.

Silicon nitride is a chemical compound of the elements silicon and nitrogen. Si
3N
4 is the most thermodynamically stable and commercially important of the silicon nitrides, and the term ″Silicon nitride″ commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot H
3PO
4. It is very hard. It has a high thermal stability with strong optical nonlinearities for all-optical applications.
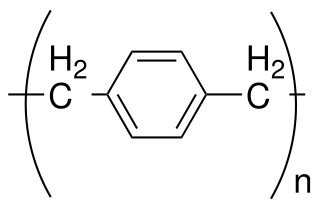
Parylene is the common name of a polymer whose backbone consists of para-benzenediyl rings −C
6H
4− connected by 1,2-ethanediyl bridges −CH
2−CH
2−. It can be obtained by polymerization of para-xylyleneH
2C=C
6H
4=CH
2.

Zirconium silicate, also zirconium orthosilicate, ZrSiO4, is a chemical compound, a silicate of zirconium. It occurs in nature as zircon, a silicate mineral. Powdered zirconium silicate is also known as zircon flour.

Hafnium(IV) oxide is the inorganic compound with the formula HfO
2. Also known as hafnium dioxide or hafnia, this colourless solid is one of the most common and stable compounds of hafnium. It is an electrical insulator with a band gap of 5.3~5.7 eV. Hafnium dioxide is an intermediate in some processes that give hafnium metal.

Plasma-enhanced chemical vapor deposition (PECVD) is a chemical vapor deposition process used to deposit thin films from a gas state (vapor) to a solid state on a substrate. Chemical reactions are involved in the process, which occur after creation of a plasma of the reacting gases. The plasma is generally created by radio frequency (RF) frequency or direct current (DC) discharge between two electrodes, the space between which is filled with the reacting gases.

Hafnon is a hafnium nesosilicate mineral, chemical formula (Hf,Zr)SiO4 or (Hf,Zr,Th,U,Y)SiO4. In natural zircon ZrSiO4 part of the zirconium is replaced by the very similar hafnium and so natural zircon is never pure ZrSiO4. A zircon with 100% hafnium substitution can be made synthetically and is hafnon.

Silicon oxynitride is a ceramic material with the chemical formula SiOxNy. While in amorphous forms its composition can continuously vary between SiO2 (silica) and Si3N4 (silicon nitride), the only known intermediate crystalline phase is Si2N2O. It is found in nature as the rare mineral sinoite in some meteorites and can be synthesized in the laboratory.
Ultra-high-temperature ceramics (UHTCs) are a type of refractory ceramics that can withstand extremely high temperatures without degrading, often above 2,000 °C. They also often have high thermal conductivities and are highly resistant to thermal shock, meaning they can withstand sudden and extreme changes in temperature without cracking or breaking. Chemically, they are usually borides, carbides, nitrides, and oxides of early transition metals.

Amorphous silicon (a-Si) is the non-crystalline form of silicon used for solar cells and thin-film transistors in LCDs.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.














