Related Research Articles

In organic chemistry, functional groups are specific substituents or moieties within molecules that may be responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of. This allows for systematic prediction of chemical reactions and behavior of chemical compounds and design of chemical syntheses. Furthermore, the reactivity of a functional group can be modified by other functional groups nearby. In organic synthesis, functional group interconversion is one of the basic types of transformations.
In chemistry, a hydride is formally the anion of hydrogen, H−. The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms, are called hydrides: water is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compound and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
In organic chemistry and biochemistry, a substituent is an atom or group of atoms which replaces one or more hydrogen atoms on the parent chain of a hydrocarbon, becoming a moiety of the resultant new molecule. The terms substituent and functional group, as well as other ones are used almost interchangeably to describe branches from a parent structure, though certain distinctions are made in the context of polymer chemistry. In polymers, side chains extend from a backbone structure. In proteins, side chains are attached to the alpha carbon atoms of the amino acid backbone.
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bond to oxygen that can dissociate to produce the H+ cation and the anion of the acid.
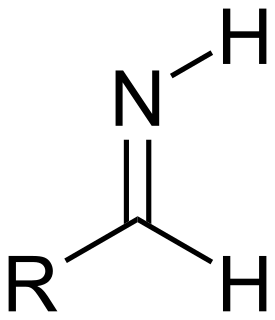
In organic chemistry, an aldimine is an imine that is an analog of an aldehyde. As such, aldimines have the general formula R–CH=N–R'. Aldimines are similar to ketimines, which are analogs of ketones.

Binary silicon-hydrogen compounds are saturated chemical compounds with the empirical formula SiHn. All contain tetrahedral silicon and terminal hydrides. They only have Si–H and Si–Si single bonds. The bond lengths are 146.0 pm for a Si–H bond and 233 pm for a Si–Si bond. The structures of the silanes are analogues of the alkanes, starting with silane, SiH
4, the analogue of methane, continuing with disilane Si
2H
6, the analogue of ethane, etc.

In chemistry, the suffix -yne is used to denote the presence of a triple bond.
Phanes are abstractions of highly complex organic molecules introduced for simplification of the naming of these highly complex molecules.
In chemistry, a hydron is the general name for a cationic form of atomic hydrogen, represented with the symbol H+
. However, virtually all chemists will call this species the "proton", which strictly speaking refers to the cation of protium, the most common isotope of hydrogen. The term "hydron", endorsed by the IUPAC, includes cations of hydrogen regardless of their isotopic composition: thus it refers collectively to protons (1H+) for the protium isotope, deuterons (2H+ or D+) for the deuterium isotope, and tritons (3H+ or T+) for the tritium isotope. Unless there is a need to explicitly address issues of isotopic composition, the term "hydron" is not used, and to refer to H+
as such is considered pedantic. Nevertheless, H+
will be referred to as the "hydron", for the sake of consistency with the article title.
Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 is the 2005 version of Nomenclature of Inorganic Chemistry. It is a collection of rules for naming inorganic compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC).
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations.
Cadmium hydride is an inorganic compound with the chemical formula (CdH
2)
n. It is a solid, known only as a thermally unstable, insoluble white powder.
Mercury(I) hydride is an inorganic compound with the empirical chemical formula HgH. It has not yet been obtained in bulk, hence its bulk properties remain unknown. However, molecular mercury(I) hydrides with the formulae HgH and Hg
2H
2 have been isolated in solid gas matrices. The molecular hydrides are very unstable toward thermal decomposition. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.

In the IUPAC nomenclature of chemistry, a parent structure, parent compound, parent name, or simply parent is the denotation for a compound consisting of an unbranched chain of skeletal atoms, or consisting of an unsubstituted monocyclic or polycyclic ring system. Parent structures bearing one or more functional groups that are not specifically denoted by a suffix are called functional parents. Names of parent structures are used in IUPAC nomenclature as basis for systematic names.

Hydrogen astatide, also known as astatine hydride, astatane, astidohydrogen or hydroastatic acid, is a chemical compound with the chemical formula HAt, consisting of an astatine atom covalently bonded to a hydrogen atom. It thus is a hydrogen halide.
Chromium(II) hydride, systematically named chromium dihydride and poly(dihydridochromium) is pale brown solid inorganic compound with the chemical formula (CrH
2)
n. Although it is thermodynamically unstable toward decomposition at ambient temperatures, it is kinetically metastable.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n. ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it known as a black, amorphous powder, which was synthesised for the first time in 2014.
References
- ↑ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 (Red Book) Par. IR-6 Parent Hydride Names and Substitutive Nomenclature - Full text PDF