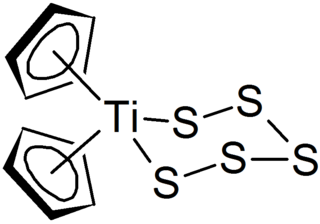Sulfide (also sulphide in British English ) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

In chemistry, catenation is the bonding of atoms of the same element into a series, called a chain. A chain or a ring shape may be open if its ends are not bonded to each other, or closed if they are bonded in a ring. The words to catenate and catenation reflect the Latin root catena, "chain".
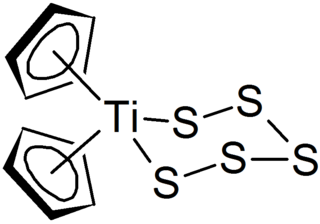
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula S2−
n. These anions are the conjugate bases of polysulfanes H2Sn. Organic polysulfides generally have the formulae R1SnR2, where R is an alkyl or aryl group.

In chemistry, triiodide usually refers to the triiodide ion, I−
3. This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been isolated, including thallium(I) triiodide (Tl+[I3]−) and ammonium triiodide ([NH4]+[I3]−). Triiodide is observed to be a red colour in solution.
Sulfur compounds are chemical compounds formed the element sulfur (S). Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.

Ammonium tetrathiomolybdate is the chemical compound with the formula (NH4)2MoS4. This bright red ammonium salt is an important reagent in the chemistry of molybdenum and has been used as a building block in bioinorganic chemistry. The thiometallate (see metallate) anion has the distinctive property of undergoing oxidation at the sulfur centers concomitant with reduction of the metal from Mo(VI) to Mo(IV).
A polysulfane is a chemical compound of formula H2Sn, where n > 1. Compounds containing 2 – 8 sulfur atoms have been isolated, longer chain compounds have been detected, but only in solution. H2S2 is colourless, higher members are yellow with the colour increasing with the sulfur content. In the chemical literature the term polysulfanes is sometimes used for compounds containing −(S)n−, e.g. organic polysulfanes R1−(S)n−R2.

The lower sulfur oxides are a group of inorganic compounds with the formula SmOn, where 2m > n. These species are often unstable and thus rarely encountered in everyday life. They are significant intermediates in the combustion of elemental sulfur. Some well characterized examples include sulfur monoxide (SO), its dimer S2O2, and a series of cyclic sulfur oxides, SnOx (x = 1, 2), based on cyclic Sn rings.

The element sulfur exists as many allotropes. In number of allotropes, sulfur is second only to carbon. In addition to the allotropes, each allotrope often exists in polymorphs delineated by Greek prefixes.
Thiophosphates (or phosphorothioates, PS) are chemical compounds and anions with the general chemical formula PS
4−xO3−
x (x = 0, 1, 2, or 3) and related derivatives where organic groups are attached to one or more O or S. Thiophosphates feature tetrahedral phosphorus(V) centers.

Disulfur is the diatomic molecule with the formula S2. It is analogous to the dioxygen molecule but rarely occurs at room temperature. This violet gas is the dominant species in hot sulfur vapors. S2 is one of the minor components of the atmosphere of Io, which is predominantly composed of SO2. The instability of S2 is usually described in the context of the double bond rule.

Disulfur monoxide or sulfur suboxide is an inorganic compound with the formula S2O, one of the lower sulfur oxides. It is a colourless gas and condenses to give a roughly dark red coloured solid that is unstable at room temperature.
In chemistry, the amino radical, ·NH2, also known as the aminyl or azanyl, is the neutral form of the amide ion. Aminyl radicals are highly reactive and consequently short-lived, like most radicals; however, they form an important part of nitrogen chemistry. In sufficiently high concentration, amino radicals dimerise to form hydrazine. While NH2 as a functional group is common in nature, forming a part of many compounds, the radical cannot be isolated in its free form.
Sulfanyl (HS•), also known as the mercapto radical, hydrosulfide radical, or hydridosulfur, is a simple radical molecule consisting of one hydrogen and one sulfur atom. The radical appears in metabolism in organisms as H2S is detoxified. Sulfanyl is one of the top three sulfur-containing gasses in gas giants such as Jupiter and is very likely to be found in brown dwarfs and cool stars. It was originally discovered by Margaret N. Lewis and John U. White at the University of California in 1939. They observed molecular absorption bands around 325 nm belonging to the system designated by 2Σ+ ← 2Πi. They generated the radical by means of a radio frequency discharge in hydrogen sulfide. HS• is formed during the degradation of hydrogen sulfide in the atmosphere of the Earth. This may be a deliberate action to destroy odours or a natural phenomenon.

Sodium polysulfide is a general term for salts with the formula Na2Sx, where x = 2 to 5. The species Sx2−, called polysulfide anions, include disulfide (S22−), trisulfide (S32−), tetrasulfide (S42−), and pentasulfide (S52−). In principle, but not in practice, the chain lengths could be longer. The salts are dark red solids that dissolve in water to give highly alkaline and corrosive solutions. In air, these salts oxidize, and they evolve hydrogen sulfide by hydrolysis.
Tellurium compounds are compounds containing the element tellurium (Te). Tellurium belongs to the chalcogen family of elements on the periodic table, which also includes oxygen, sulfur, selenium and polonium: Tellurium and selenium compounds are similar. Tellurium exhibits the oxidation states −2, +2, +4 and +6, with +4 being most common.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.

Spectroelectrochemistry (SEC) is a set of multi-response analytical techniques in which complementary chemical information is obtained in a single experiment. Spectroelectrochemistry provides a whole vision of the phenomena that take place in the electrode process. The first spectroelectrochemical experiment was carried out by Theodore Kuwana, PhD, in 1964.

Disulfidobis(tricarbonyliron), or Fe2(μ-S2)(CO)6, is an organometallic molecule used as a precursor in the synthesis of iron-sulfur compounds. Popularized as a synthetic building block by Dietmar Seyferth, Fe2(μ-S2)(CO)6 is commonly used to make mimics of the H-cluster in [FeFe]-hydrogenase. Much of the reactivity of Fe2(μ-S2)(CO)6 proceeds through its sulfur-centered dianion, [Fe2(μ-S)2(CO)2]2-.