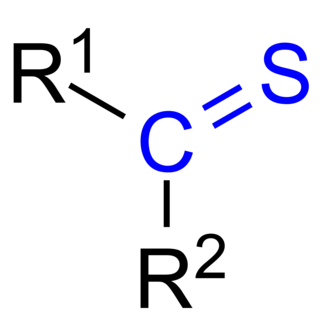In chemistry, an alkene is a hydrocarbon that contains a carbon–carbon double bond.

Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean-Antoine-Claude Chaptal in 1790 when it was found that nitrogen was present in nitric acid and nitrates. Antoine Lavoisier suggested instead the name azote, from the Greek ἀζωτικός "no life", as it is an asphyxiant gas; this name is instead used in many languages, such as French, Italian, Russian, Romanian, Portuguese and Turkish, and appears in the English names of some nitrogen compounds such as hydrazine, azides and azo compounds.

In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated.
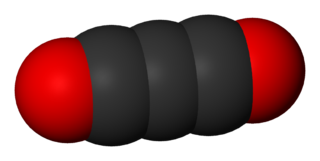
Carbon suboxide, or tricarbon dioxide, is an oxide of carbon with chemical formula C
3O
2 or O=C=C=C=O. Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons O=Cn=O, which also includes carbon dioxide (CO2) and pentacarbon dioxide (C
5O
2). Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions.
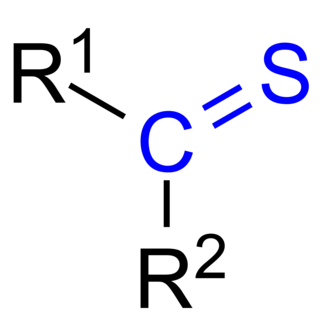
Thioketones (also known as thiones or thiocarbonyls) are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix "thio-" in the name of the functional group. Unhindered alkylthioketones typically tend to form polymers or rings.

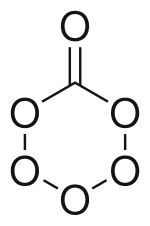
Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups Cs, D3h, and C2v. The C2v state, consisting of a dioxirane has been shown to be the ground state of the molecule. Carbon trioxide should not be confused with the stable carbonate ion (CO32−).

Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp2-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of aromatic organosulfur compounds. A few 1,2-dithietes have been isolated.

In chemistry, dioxirane is a compound with formula CH
2O
2, whose molecule consists of a ring with one carbon and two oxygen atoms, and two hydrogen atoms attached to the carbon. It is a heterocyclic compound, the smallest cyclic organic peroxide.

Iodine pentoxide is the chemical compound with the formula I2O5. This iodine oxide is the anhydride of iodic acid, and the only stable oxide of iodine. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:

An oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide (CO2). Many other stable (practically if not thermodynamically) or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide (C3O2 or O=C=C=C=O) and mellitic anhydride (C12O9).

In chemistry, isomers are molecules or polyatomic ions with identical molecular formulas — that is, same number of atoms of each element — but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.

Carbon tetroxide is a highly unstable oxide of carbon with formula CO
4. It was proposed as an intermediate in the O-atom exchange between carbon dioxide and oxygen at high temperatures. The C2v isomer, which is -138 kJ mol−1 more stable than the D2d isomer, was first detected in electron-irradiated ices of carbon dioxide via infrared spectroscopy.

Dihydroxymethylidene is a chemical compound with formula C(OH)2. It is an unstable tautomer of formic acid. There is no evidence that this compound exists in solution, but the molecule has been detected in the gas phase. Many related carbenes are known, although they are often transient.

Disulfur monoxide or sulfur suboxide is an inorganic compound with formula S2O. It is one of the lower sulfur oxides. It is a colourless gas and condenses to give a pale coloured solid that is unstable at room temperature. It is a bent molecule with an S−S−O angle of 117.88°, S−S bond length of 188.4 pm, and S−O bond length of 146.5 pm.

Disulfur dioxide, dimeric sulfur monoxide or SO dimer is an oxide of sulfur with the formula S2O2. The solid is unstable with a lifetime of a few seconds at room temperature.
Uranium hexoxide is an unusual, theoretically possible compound of uranium in which the uranium atom would be attached to six oxygen atoms. It would be an unprecedented example of an element in the +12 oxidation state; for comparison, the highest known oxidation state is +9 for iridium in the cation IrO+
4.

Carbon pentaoxide or carbon pentoxide is an unstable molecular oxide of carbon. The molecule has been produced and studied at cryogenic temperatures. The molecule is important in atmospheric chemistry and in the study of cold ices in the outer solar system and interstellar space. The substance could form and be present on Ganymede or Triton, moons in the outer solar system.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.

Tricarbon monoxide C3O is a reactive radical oxocarbon molecule found in space, and which can be made as a transient substance in the laboratory. It can be trapped in an inert gas matrix or made as a short lived gas. C3O can be classified as a ketene or an oxocumulene a kind of heterocumulene.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.