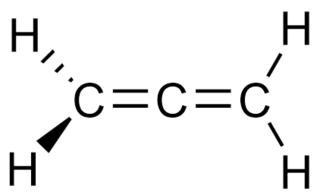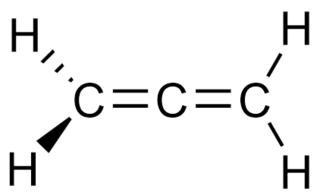
Allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding.

The Pauson–Khand reaction is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone. Ihsan Ullah Khand (1935-1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow. Pauson and Khand's initial findings were intermolecular in nature, but starting a decade after the reaction's discovery, many intramolecular examples have been highlighted in both synthesis and methodology reports. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient, enhancing reactivity and yield via utilizing different chiral auxiliaries for stereo induction, main group transition-metals, and additives.

In chemistry, a phosphaalkyne is an organophosphorus compound containing a triple bond between phosphorus and carbon with the general formula R-C≡P. Phosphaalkynes are the heavier congeners of nitriles, though, due to the similar electronegativities of phosphorus and carbon, possess reactivity patterns reminiscent of alkynes. Due to their high reactivity, phosphaalkynes are not found naturally on earth, but the simplest phosphaalkyne, phosphaethyne (H-C≡P) has been observed in the interstellar medium.

In chemistry, a polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (−C≡C−)
n with n greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds (−CR=CR′−). They are also sometimes referred to as oligoynes, or carbinoids after "carbyne" (−C≡C−)
∞, the hypothetical allotrope of carbon that would be the ultimate member of the series. The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today.

Germabenzene (C5H6Ge) is the parent representative of a group of chemical compounds containing in their molecular structure a benzene ring with a carbon atom replaced by a germanium atom. Germabenzene itself has been studied theoretically, and synthesized with a bulky 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl or Tbt group. Also, stable naphthalene derivatives do exist in the laboratory such as the 2-germanaphthalene-containing substance represented below. The germanium to carbon bond in this compound is shielded from potential reactants by a Tbt group. This compound is aromatic just as the other carbon group representatives silabenzene and stannabenzene.
The Wurtz–Fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. This modification of the Wurtz reaction is considered a separate process and is named for both scientists.
The Riemschneider thiocarbamate synthesis converts alkyl or aryl thiocyanates to thiocarbamates under acidic conditions, followed by hydrolysis with ice water. The reaction was discovered by the German chemist Randolph Riemschneider in 1951 as a more efficient method to produce thiocarbamates. Some references spell the name Riemenschneider.

The Achmatowicz reaction, also known as the Achmatowicz rearrangement, is an organic synthesis in which a furan is converted to a dihydropyran. In the original publication by the Polish Chemist Osman Achmatowicz Jr. in 1971 furfuryl alcohol is reacted with bromine in methanol to 2,5-dimethoxy-2,5-dihydrofuran which rearranges to the dihydropyran with dilute sulfuric acid. Additional reaction steps, alcohol protection with methyl orthoformate and boron trifluoride) and then ketone reduction with sodium borohydride produce an intermediate from which many monosaccharides can be synthesised.

Distonic ions are chemical species that contain two ionic charges on the same molecule, separated by two or more carbon or heteroatoms. A feature of distonic radical ions is that their charges and radical sites are in different locations, unlike regular radicals where the formal charge and unpaired electron are in the same location. These molecular species are created by ionization of either zwitterions or diradicals; ultimately, a neutral molecule loses an electron. Through experimental research distonic radicals have been found to be extremely stable gas phase ions and can be separated into different classes depending on the inherent features of the charged portion of the ion.

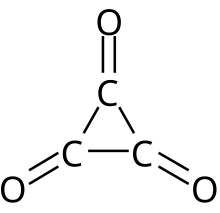
An oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide (CO2). Many other stable (practically if not thermodynamically) or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide (C3O2 or O=C=C=C=O) and mellitic anhydride (C12O9).
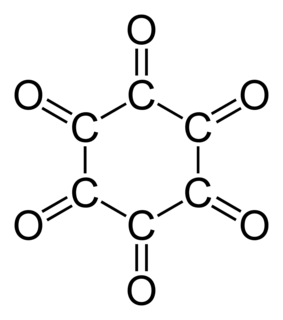
Cyclohexanehexone, also known as hexaketocyclohexane and triquinoyl, is an organic compound with formula C6O6, the sixfold ketone of cyclohexane. It is an oxide of carbon, a hexamer of carbon monoxide.

Cyclopentanepentone, also known as leuconic acid, is a hypothetical organic compound with formula C5O5, the fivefold ketone of cyclopentane. It would be an oxide of carbon (an oxocarbon), indeed a pentamer of carbon monoxide.

Cyclobutanetetrone, also called tetraoxocyclobutane, is an organic compound with formula C4O4 or (CO)4, the fourfold ketone of cyclobutane. It would be an oxide of carbon, indeed a tetramer of carbon monoxide.
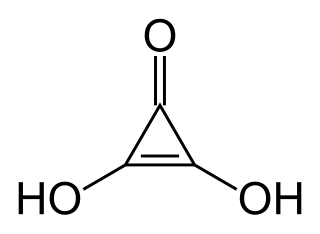
Deltic acid or dihydroxycyclopropenone is a chemical substance with the chemical formula C3O(OH)2. It can be viewed as a ketone and double alcohol of cyclopropene. At room temperature, it is a stable white solid, soluble in diethyl ether, that decomposes (sometimes explosively) between 140 °C and 180 °C, and reacts slowly with water.
David Markham Lemal is the Albert W. Smith Professor of Chemistry Emeritus and Research Professor of Chemistry at Dartmouth College. He received an A.B. degree (summa) from Amherst College in 1955 and a Ph.D. in Chemistry from Harvard University in 1959. At Harvard he worked with R. B. Woodward on deoxy sugars and a synthesis of the alkaloid yohimbine.
In organic chemistry, the Fujiwara–Moritani reaction is a type of cross coupling reaction where an aromatic C-H bond is directly coupled to an olefinic C-H bond, generating a new C-C bond. This reaction is performed in the presence of a transition metal, typically palladium. The reaction was discovered by Yuzo Fujiwara and Ichiro Moritani in 1967. An external oxidant is required to this reaction to be run catalytically. Thus, this reaction can be classified as a C-H activation reaction, an oxidative Heck reaction, and a C-H olefination. Surprisingly, the Fujiwara–Moritani reaction was discovered before the Heck reaction.
In organometallic chemistry, palladacycle, as a class of metallacycles, refers to complexes containing at least one carbon-palladium bond. Palladacycles are invoked as intermediates in catalytic or palladium mediated reactions. They have been investigated as pre-catalysts for homogeneous catalysis and synthesis.

Cyclohexanehexathione is a cyclic covalent compound consisting of a six-carbon ring with a sulfur bonded to each. It has been synthesized by neutralization of its monoanion in a mass spectrometer. This compound is the thioketone analog of cyclohexanehexone; that oxygen variant is expected to be substantially less stable. Synthesis of C6S6 by photolysis or pyrolysis to extrude three equivalents of carbon monoxide from a precursor containing adjacent pairs of sulfurs as cyclic dithiocarbonate units gave what is more likely a different valence isomer, as various dithiete-containing structures are predicted to be more stable than the hexathione form.

The Mizoroki−Heck coupling of aryl halides and alkenes to form C(sp2)–C(sp2) bonds has become a staple transformation in organic synthesis, owing to its broad functional group compatibility and varied scope. In stark contrast, the palladium-catalyzed reductive Heck reaction has received considerably less attention, despite the fact that early reports of this reaction date back almost half a century. From the perspective of retrosynthetic logic, this transformation is highly enabling because it can forge alkyl–aryl linkages from widely available alkenes, rather than from the less accessible and/or more expensive alkyl halide or organometallic C(sp3) synthons that are needed in a classical aryl/alkyl cross-coupling.
F. Dean Toste is the Gerald E. K. Branch Distinguished Professor of Chemistry at the University of California, Berkeley and Faculty Scientist at the Chemical Sciences Division of Lawrence Berkeley National Lab. He is a prominent figure in the field of organic chemistry and is best known for his contributions to gold chemistry and asymmetric ion-pairing catalysis. Toste was elected a member of the National Academy of Sciences in 2020, and a member of the American Academy of Arts and Sciences in 2018.